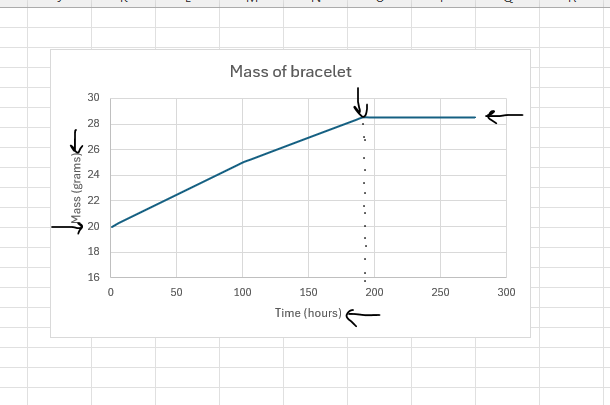
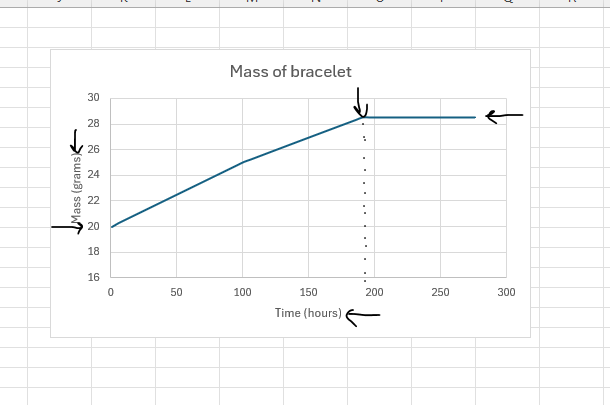
PC1.2 CP/PC1.4 ENH Review Conservation of a Mass
star
star
star
star
star
Last updated 9 months ago
11 questions
Untitled Section
Required
12
Required
4
Interpreting Data from Chemical Reactions
Required
5
Required
5
Required
11
Conservation of Mass Problems
Required
2
Required
2
Required
2
Required
2
Required
1
Required
1