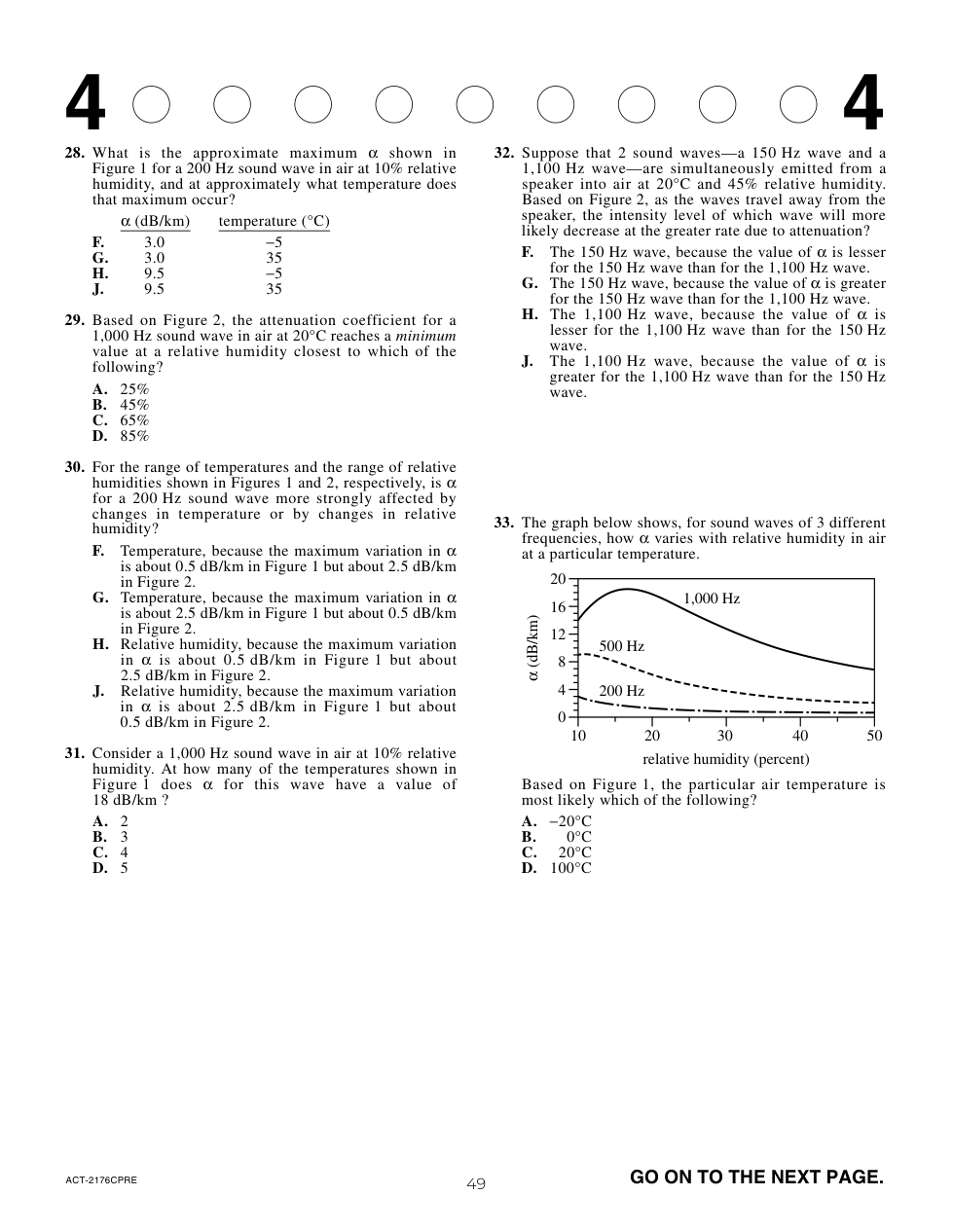
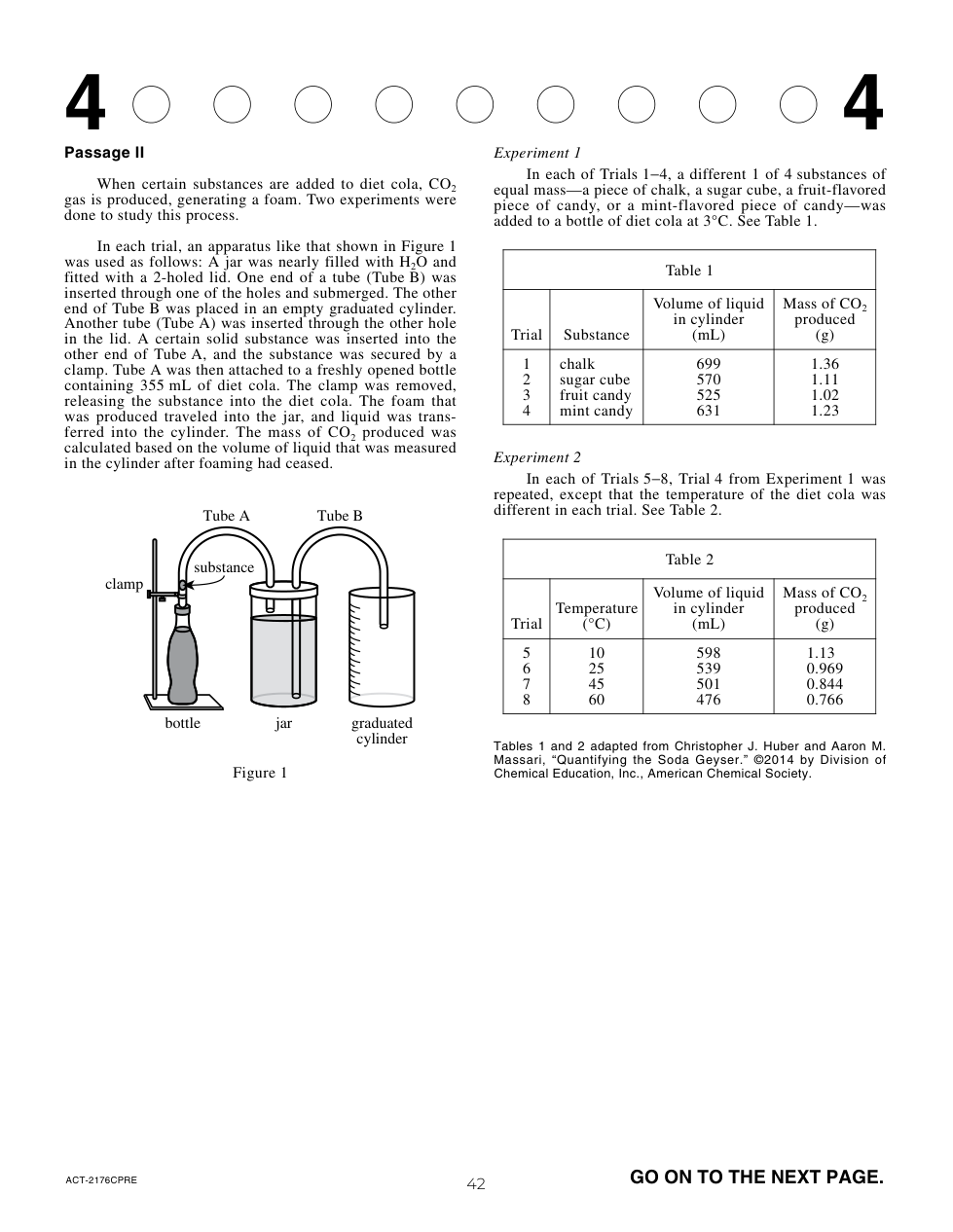
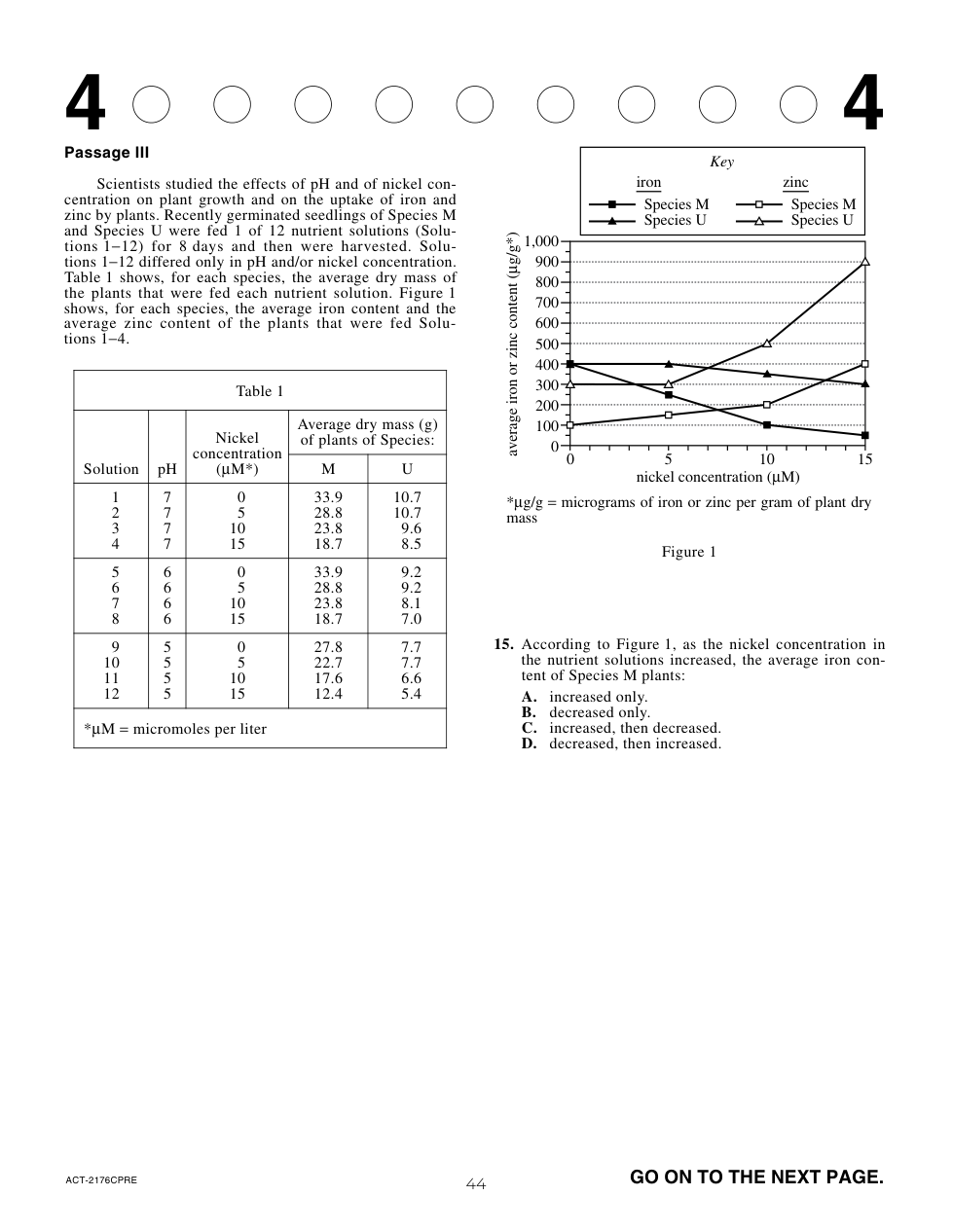
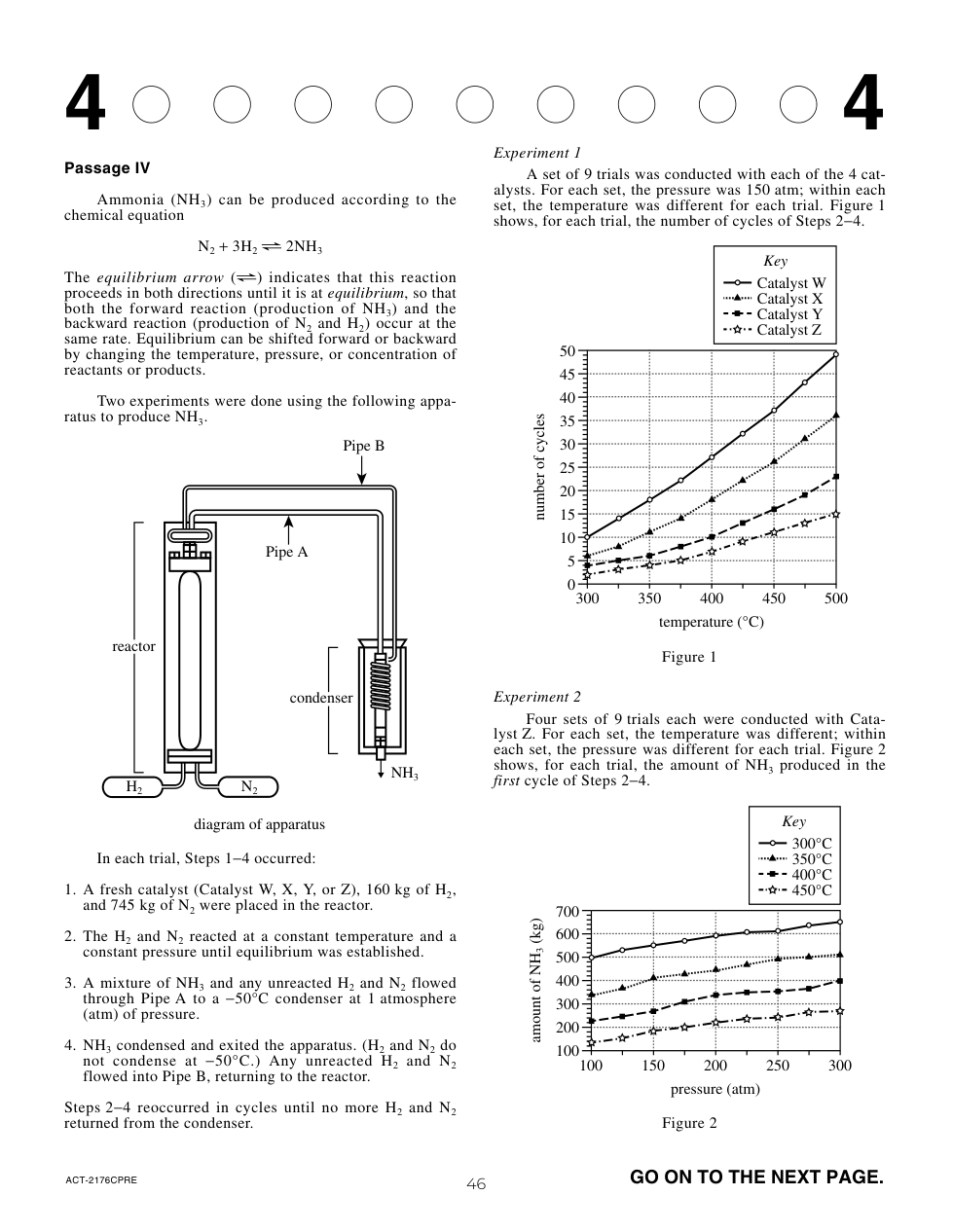
Suppose a researcher observed that wind speeds
greater than 80 miles per hour are needed to move the
rocks in the playa. This observation is consistent with
which of the scientists’ explanations?
F. Scientists 1 and 2 only
G. Scientists 1 and 3 only
H. Scientists 2 and 3 only
J. Scientists 1, 2, and 3