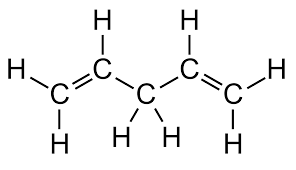
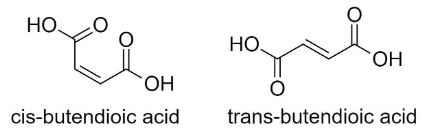
RI_11 Alkenes
star
star
star
star
star
Last updated about 1 year ago
5 questions
Required
1
Alkanes and alkenes are both non-polar due to the C-C and C-H bonds present.
Explain why alkenes are more reactive than alkanes.
Alkanes and alkenes are both non-polar due to the C-C and C-H bonds present.
Explain why alkenes are more reactive than alkanes.
Required
1
Required
1