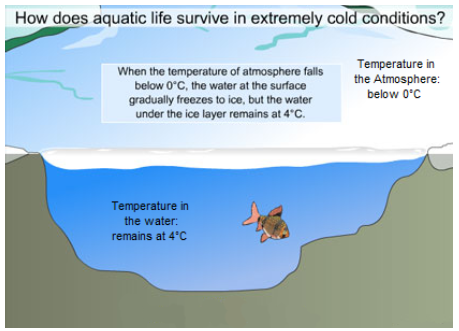
Maria, after finding no cold sodas in the refrigerator, placed a can of soda in the freezer. She then proceeded to check her social networking site and forgot about the soda. Later that evening her brother went to get some ice and it was brown. Maria’s soda can had split open. What is the best explanation for what happened?