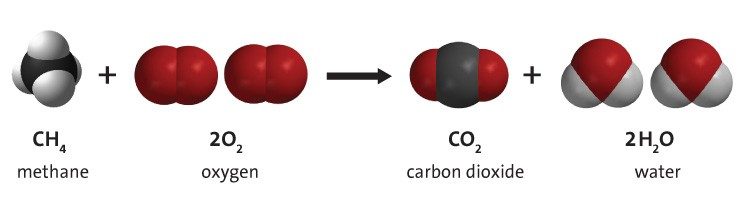
Chemical Changes
star
star
star
star
star
Last updated over 1 year ago
18 questions
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
| Draggable item | arrow_right_alt | Corresponding Item |
|---|---|---|
Ice melting into water | arrow_right_alt | No chemical change |
Iron rusting over time | arrow_right_alt | Chemical change |
Wood burning into ashes | arrow_right_alt | Chemical change |
| Draggable item | arrow_right_alt | Corresponding Item |
|---|---|---|
Cutting a piece of cake | arrow_right_alt | Physical change |
A green leaf turning yellow in autumn | arrow_right_alt | Chemical change |
The formation of water from hydrogen and oxygen | arrow_right_alt | Chemical reaction |