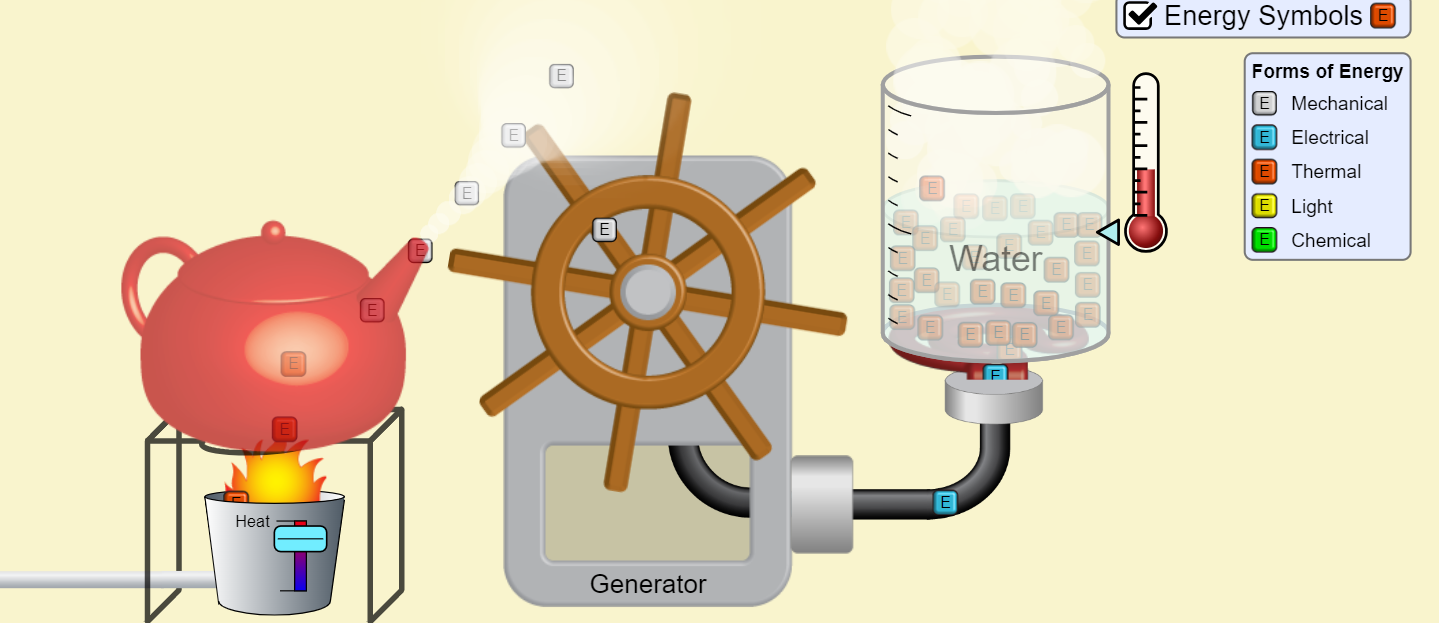
Lesson 2.6 Energy Transfer and Heat Capacity Review
star
star
star
star
star
Last updated 9 months ago
26 questions
Untitled Section
23
4
Revewing Heat Capacity
1
1
1
1
1
10
4
6
Reviewing Energy Transfer
7
5
2
2
1
1
2
2
| Draggable item | arrow_right_alt | Corresponding Item |
|---|---|---|
ΔQ | arrow_right_alt | the energy of moving particles |
joule | arrow_right_alt | Energy used to move particles in the presence of a force |
Attractive force | arrow_right_alt | pulls particles together |
Absolute Zero | arrow_right_alt | pulls particles apart |
calorie | arrow_right_alt | Energy associated with attractive forces |
Chemical Potential Energy | arrow_right_alt | Energy that enters or leaves a system |
Conduction | arrow_right_alt | Energy transfer by particles colliding with each other |
Calorie | arrow_right_alt | Energy transfer by light energy being absorbed or released |
C | arrow_right_alt | energy associated with attractive forces between atoms or molecules |
Kinetic Energy | arrow_right_alt | energy is entering the system |
Heat | arrow_right_alt | energy is leaving the system |
Repulsive force | arrow_right_alt | energy can be neither created or destroyed |
Radiation | arrow_right_alt | Measures the average kinetic energy of the particles in the system |
Law of Conservation of Energy | arrow_right_alt | the amount of energy needed to increase the temperature of one gram of a substance by one degree Celcius |
Temperature | arrow_right_alt | the temperature at which no particles are moving in a system |
Endothermic process | arrow_right_alt | temperature scale that starts at absolute zero |
Potential Energy | arrow_right_alt | symbol for change in |
Specific Heat Capacity | arrow_right_alt | heat the flowed into or out of the system |
Δ | arrow_right_alt | change in temperature of the system |
Work | arrow_right_alt | specific heat capacity of a substance |
Kelvin | arrow_right_alt | S.I (metric) unit of energy |
Exothermic process | arrow_right_alt | imperial system unit of energy |
ΔT | arrow_right_alt | Food calories- is equal to 1000 calories |
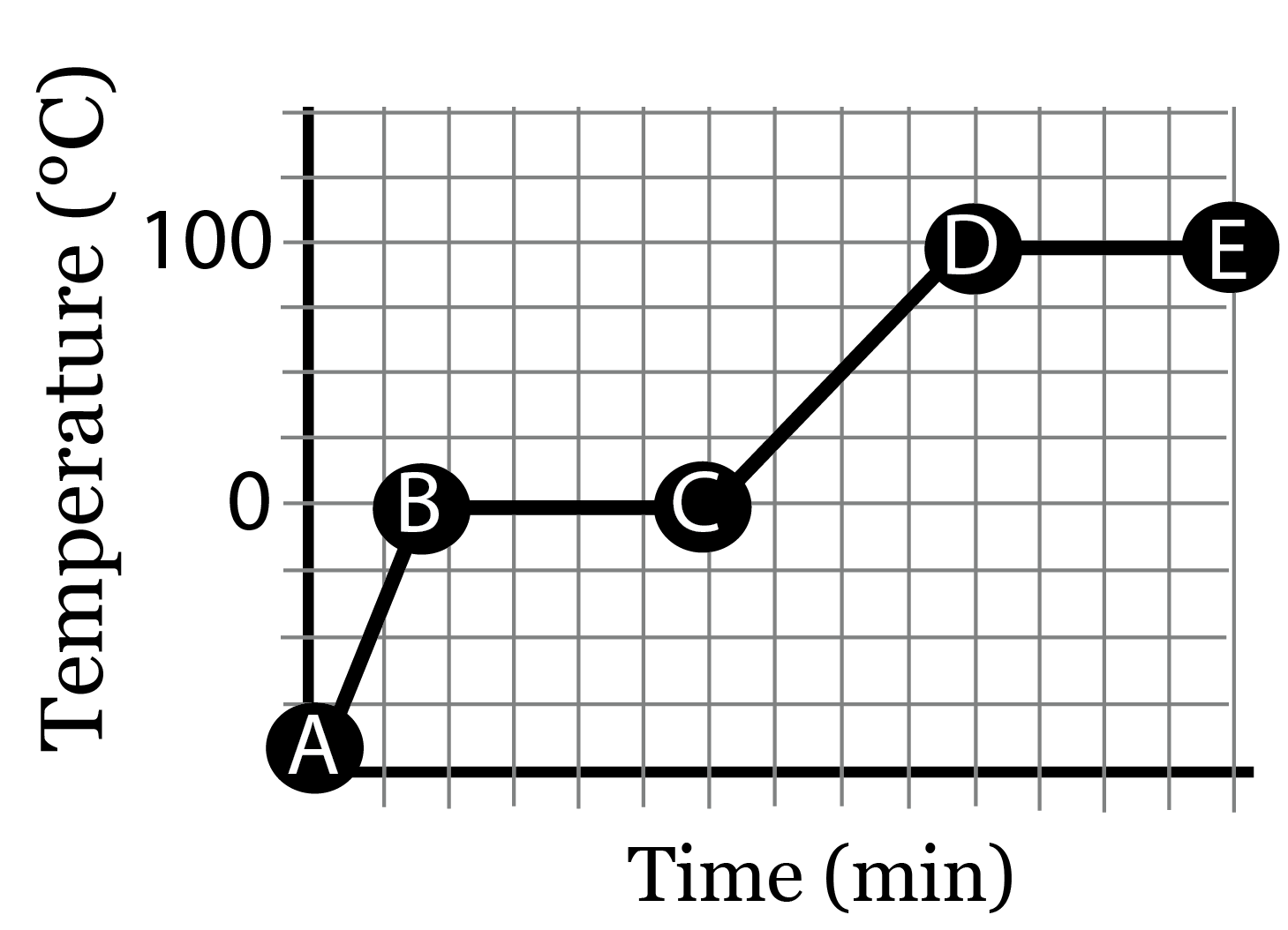
Substance 1 | Substance 2 | |
|---|---|---|
Lowest Melting Point | ||
Lowest Boiling Point | ||
Lowest Heat Capacity | ||
Most temperature change for 1 unit of heat | ||
Requires most heat to melt | ||
Requires most heat to change temperature of liquid |