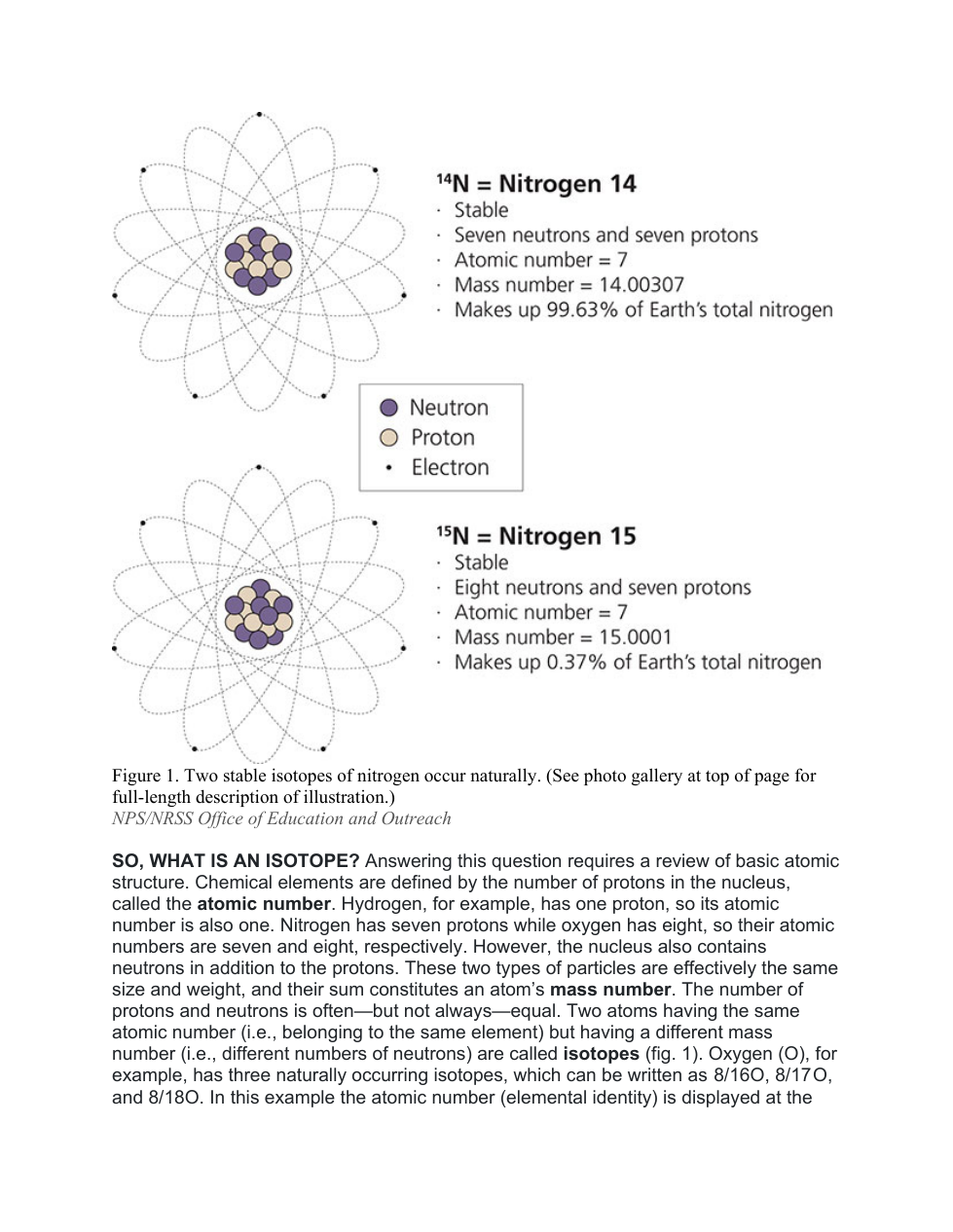
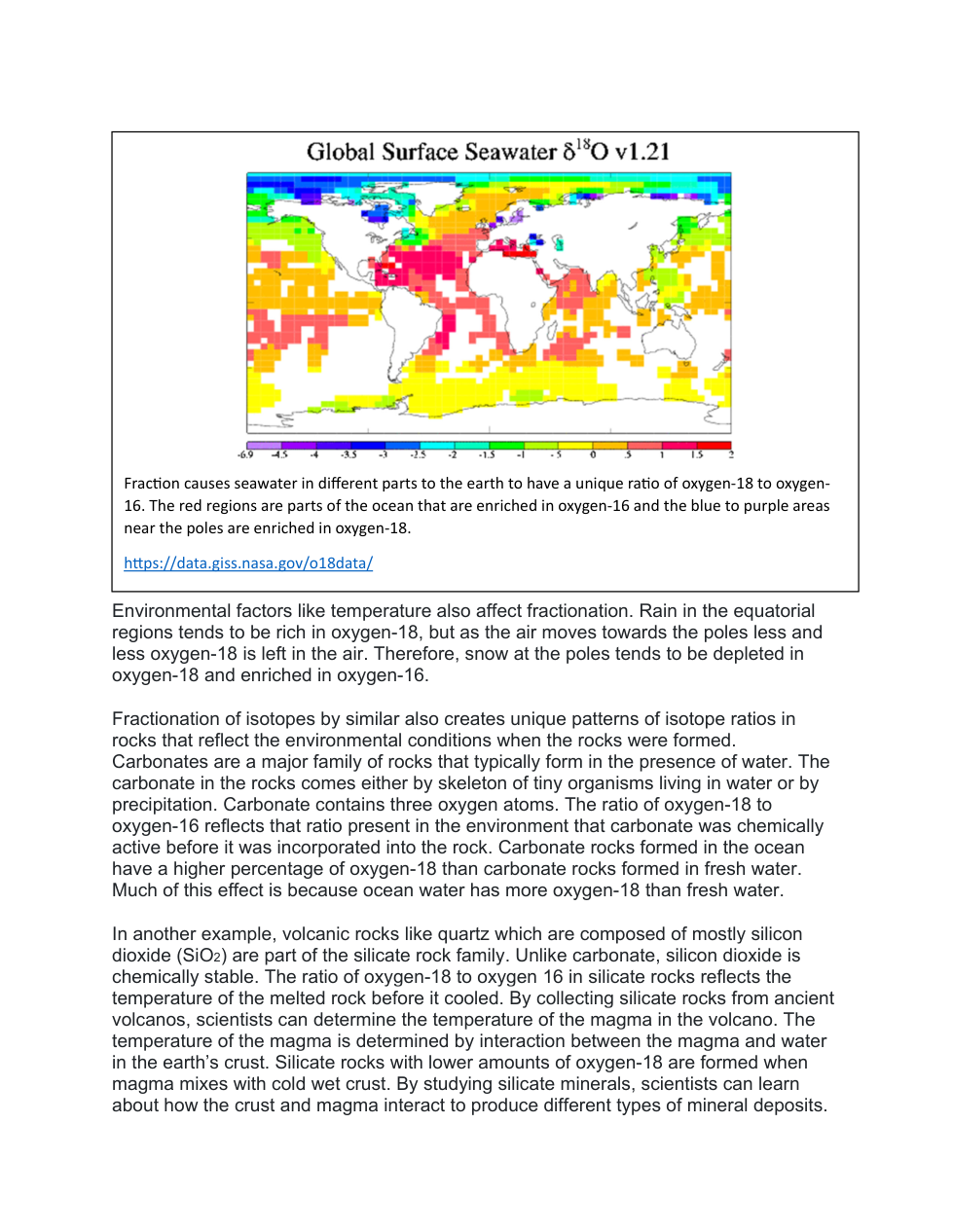
An atom is said to "decay" when the contents of the nucleus change and release radiation. Radiation can be both energy and particles. If an unstable atom releases particles in a way that the atomic number has changed, the number of ___________ in the nucleus has changed.