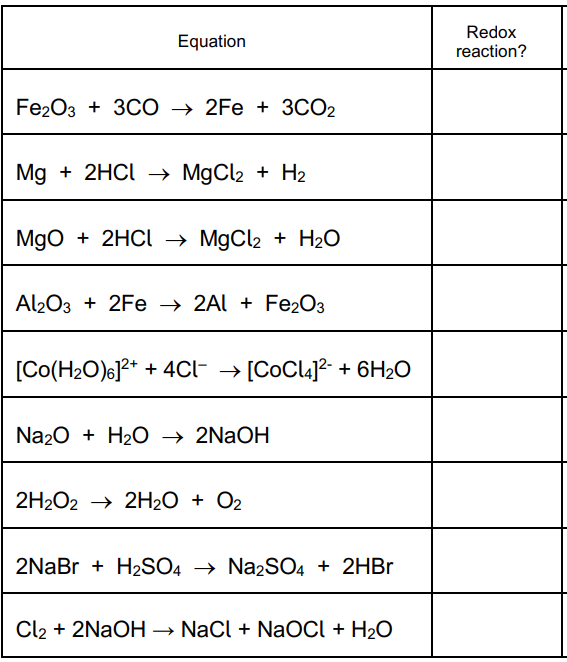
An oxidising agent (____________ ) is the substance that does the oxidising (i.e. takes away the electrons from the substance that is oxidised, and so the oxidising agent is itself ____________ ).
An reducing agent (______________ ) is the substance that does the reducing (i.e. donates the electrons to the substance that is reduced, and so the reducing agent is itself _____________ )
A disproportionation reaction is one in which a species is both _____________ and reduced.