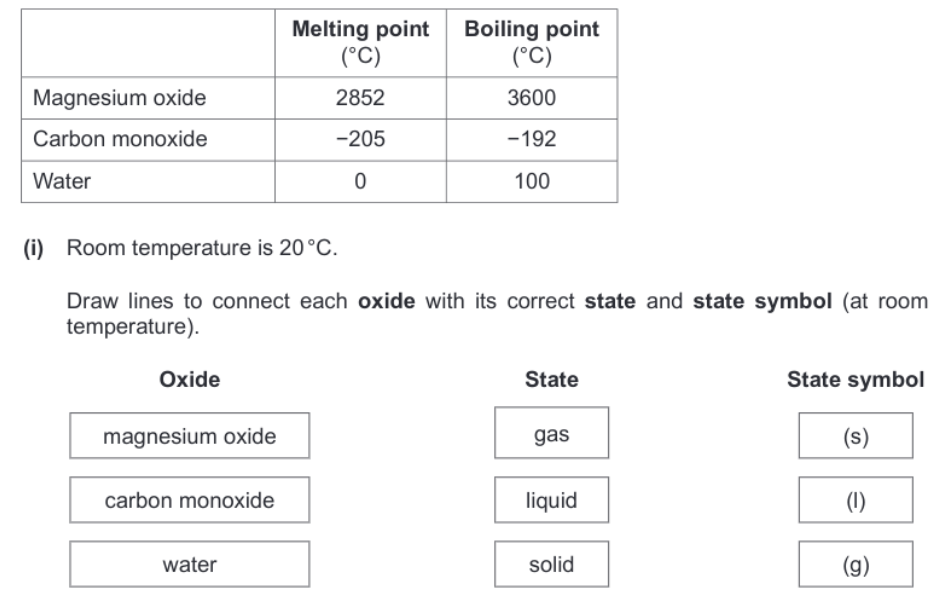
States of matter
star
star
star
star
star
Last updated 8 months ago
15 questions
1
11
13
1
2
1
1
1
3
4
8
1
3
3
3
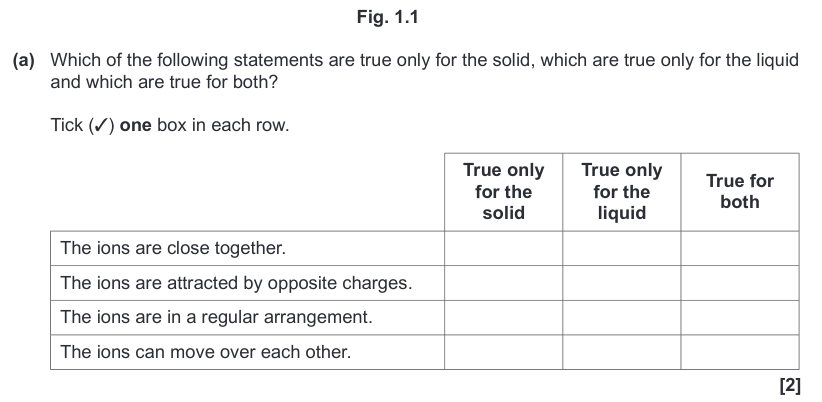
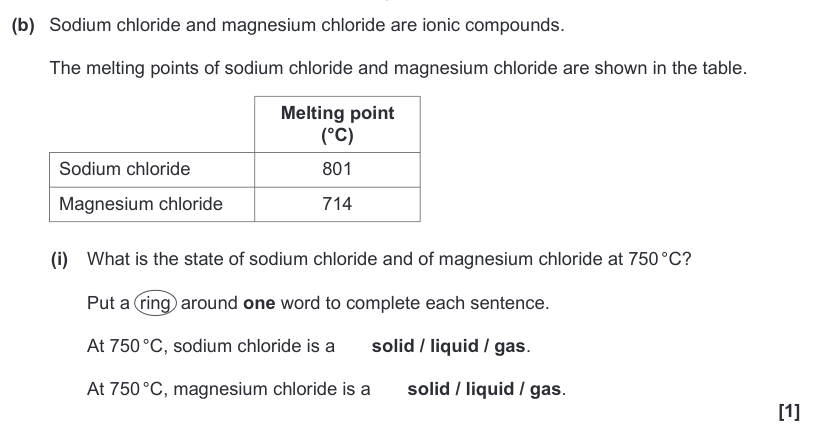
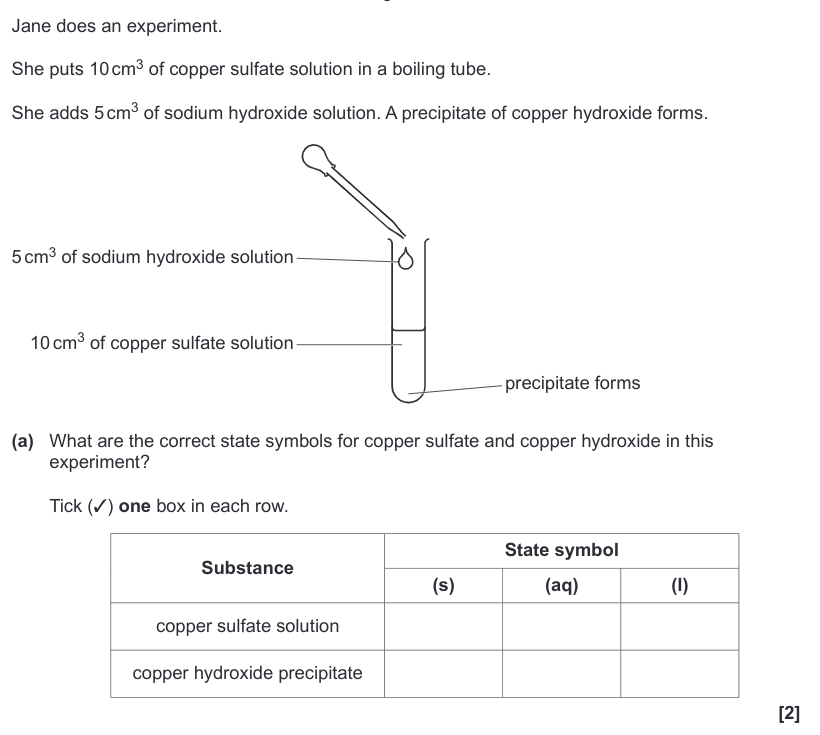


| Draggable item | arrow_right_alt | Corresponding Item |
|---|---|---|
measure 100 cm3 of water | arrow_right_alt | mass balance |
add potassium chloride | arrow_right_alt | thermometer |
measure temperature | arrow_right_alt | spatula |
measure mass | arrow_right_alt | measuring cylinder |