RGSHW Year 12 REDOX
star
star
star
star
star
Last updated 3 months ago
26 questions
2
4
3
10
1
1
8
7
1
1
1
10
5
7
4
4
5
7
2
1
5
6
6
4
6
3
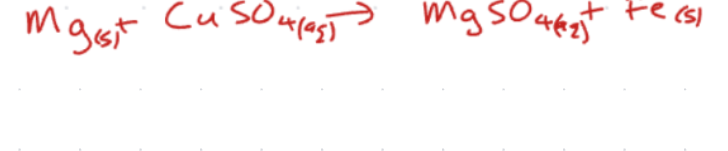
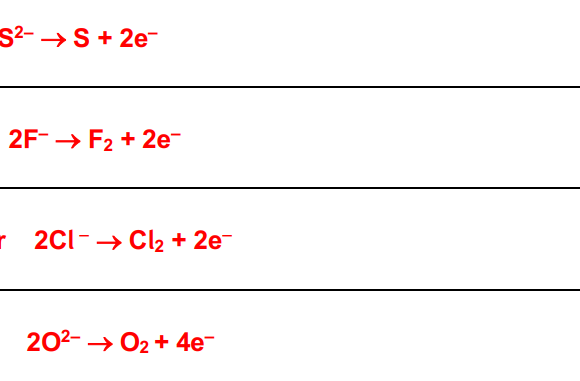

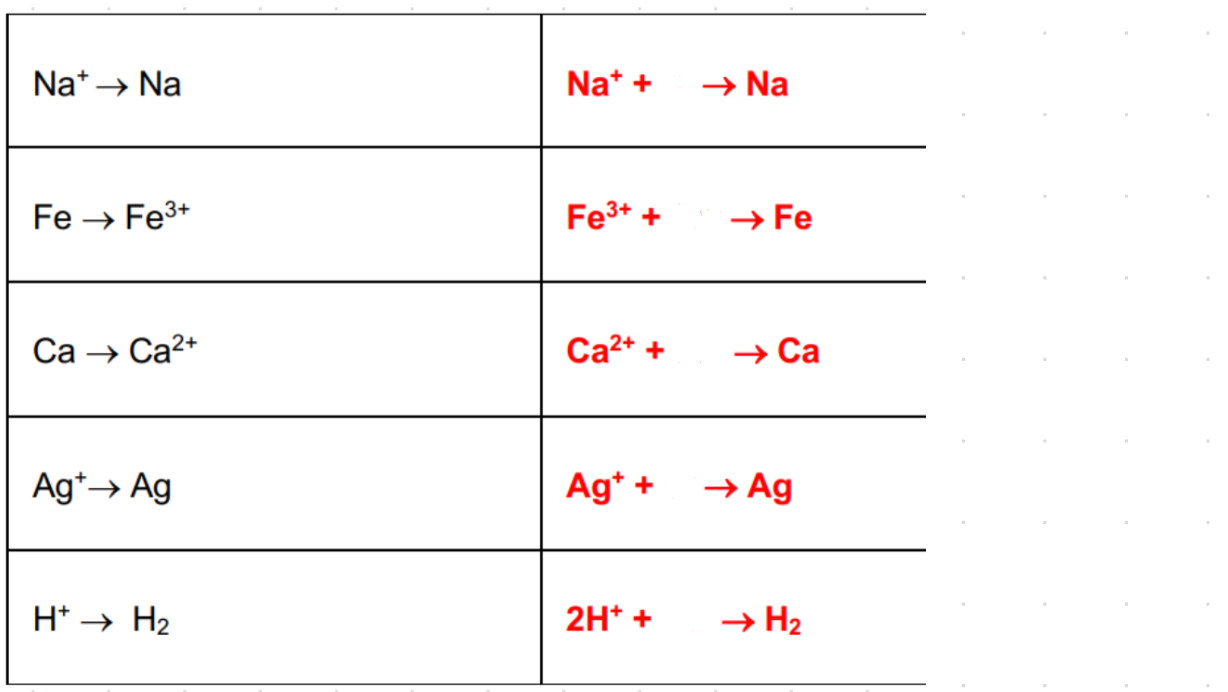
-2 | -1 | 0 | +1 | +2 | +3 | +4 | +5 | |
|---|---|---|---|---|---|---|---|---|
Br₂ | ||||||||
HBr | ||||||||
HBrO₃ | ||||||||
HOBr | ||||||||
NaBr | ||||||||
KBr | ||||||||
MgBr₂ | ||||||||
AlBr₃ |
-2 | 0 | +2 | +4 | +6 | |
|---|---|---|---|---|---|
H₂S | |||||
S₈ | |||||
SO₂ | |||||
SO₃ | |||||
H₂SO₄ | |||||
Na₂SO₄ | |||||
MgSO₄ |
-3 | 0 | +2 | +3 | +4 | +5 | |
|---|---|---|---|---|---|---|
N₂ | ||||||
NaNO₃ | ||||||
NH₃ | ||||||
NO₃⁻ | ||||||
HNO₃ | ||||||
Mg(NO₃)₂ | ||||||
NH₄⁺ | ||||||
NO | ||||||
NO₂ | ||||||
NO₂⁻ |
-1 | 0 | +1 | |
|---|---|---|---|
H₂ | |||
H₂O | |||
NaH | |||
H₂O₂ | |||
CH₄ |
-2 | -1 | 0 | +1 | +2 | |
|---|---|---|---|---|---|
O₂ | |||||
H₂O | |||||
H₂O₂ | |||||
Na₂O | |||||
MgO | |||||
H₂SO₄ | |||||
F₂O |
| Draggable item | arrow_right_alt | Corresponding Item |
|---|---|---|
CaO | arrow_right_alt | 2+ |
NO₂ | arrow_right_alt | 2- |
CaO | arrow_right_alt | 4+ |
O₂ | arrow_right_alt | 0 |
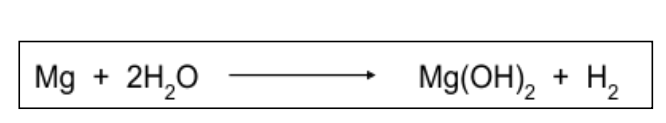

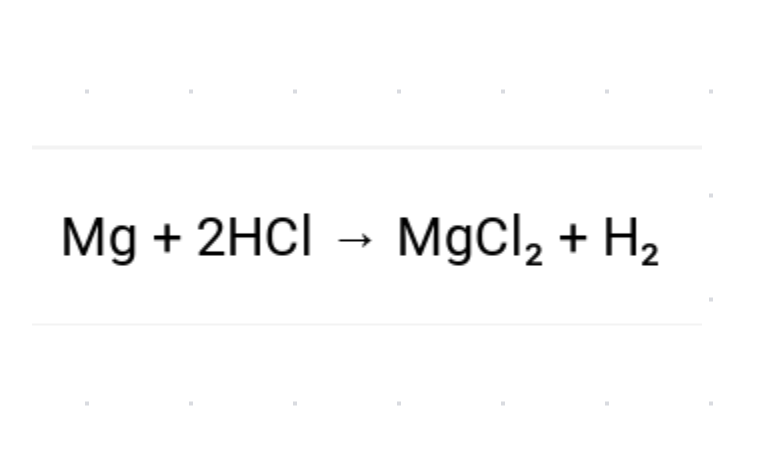
| Draggable item | arrow_right_alt | Corresponding Item |
|---|---|---|
Ca(NO₃)₂ | arrow_right_alt | 2+ |
Ca(NO₃)₂ | arrow_right_alt | 5+ |
Ca(NO₃)₂ | arrow_right_alt | 2- |