Explain why you cannot squash liquid water.
Particles are __________ together so cannot be put closer together
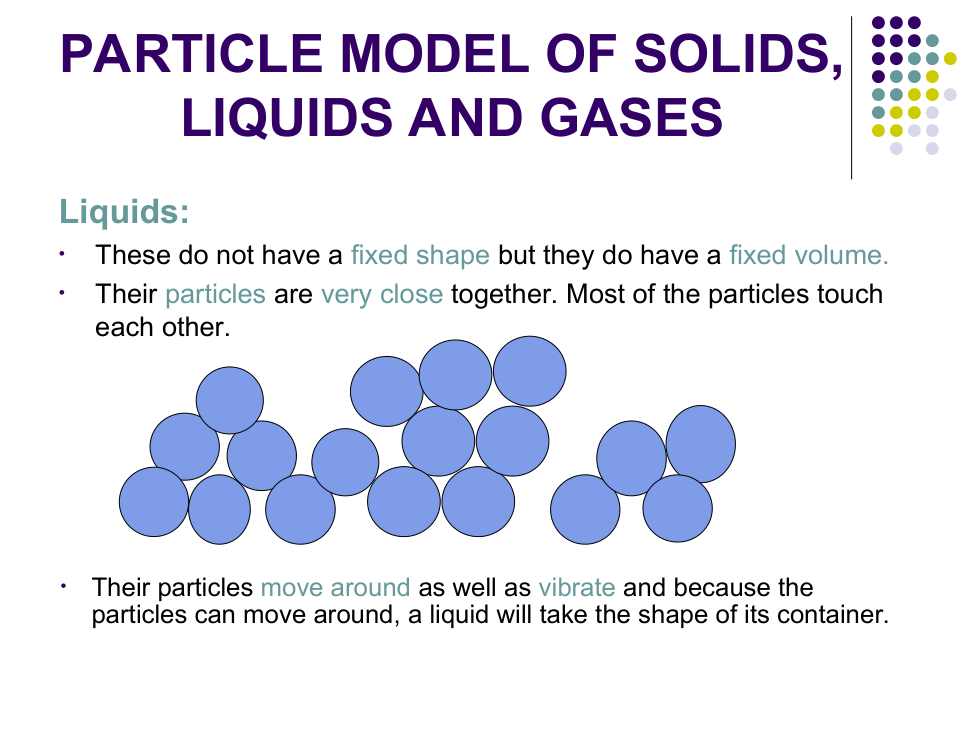
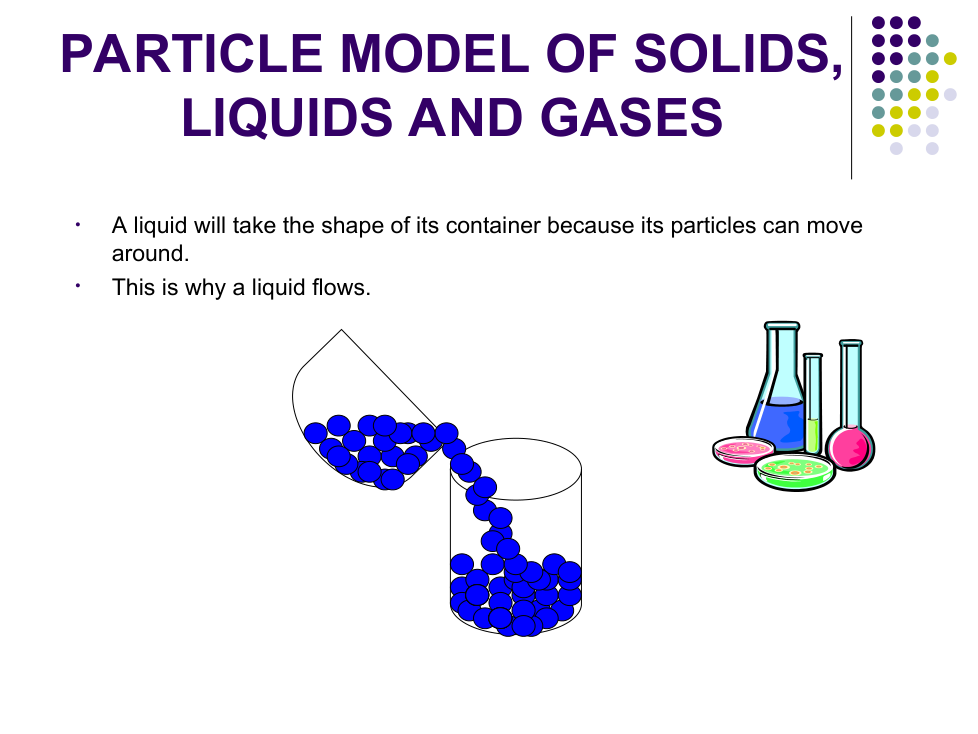
b) Explain why liquid water changes shape.
Particles can _________ around
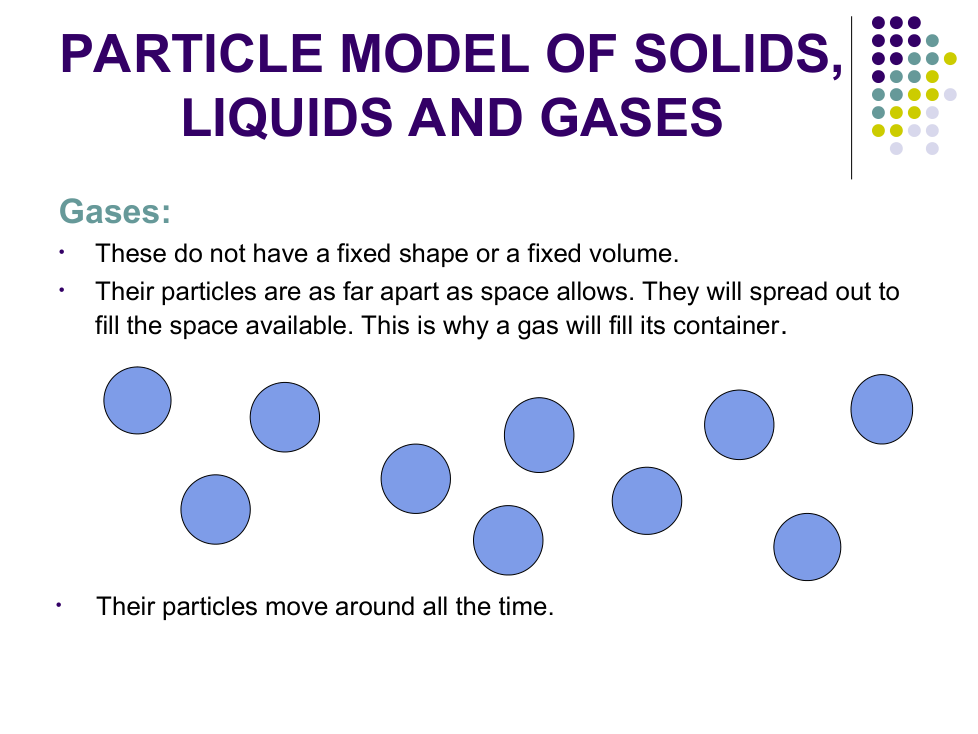
Why do liquids and gases take the shape of their container?
Particles can _________ around
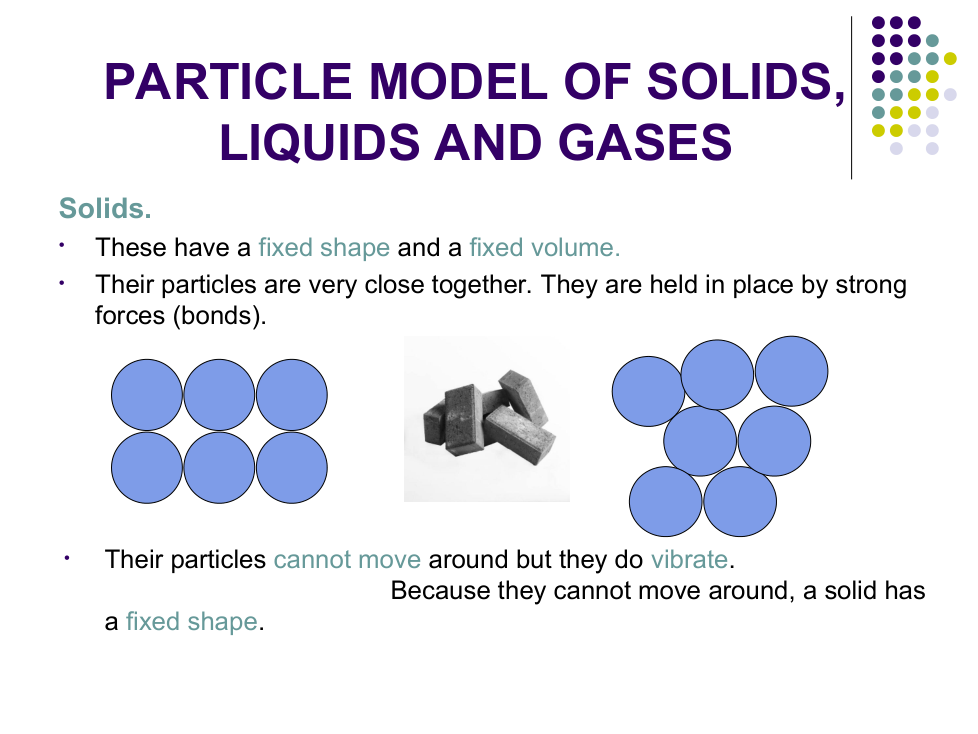
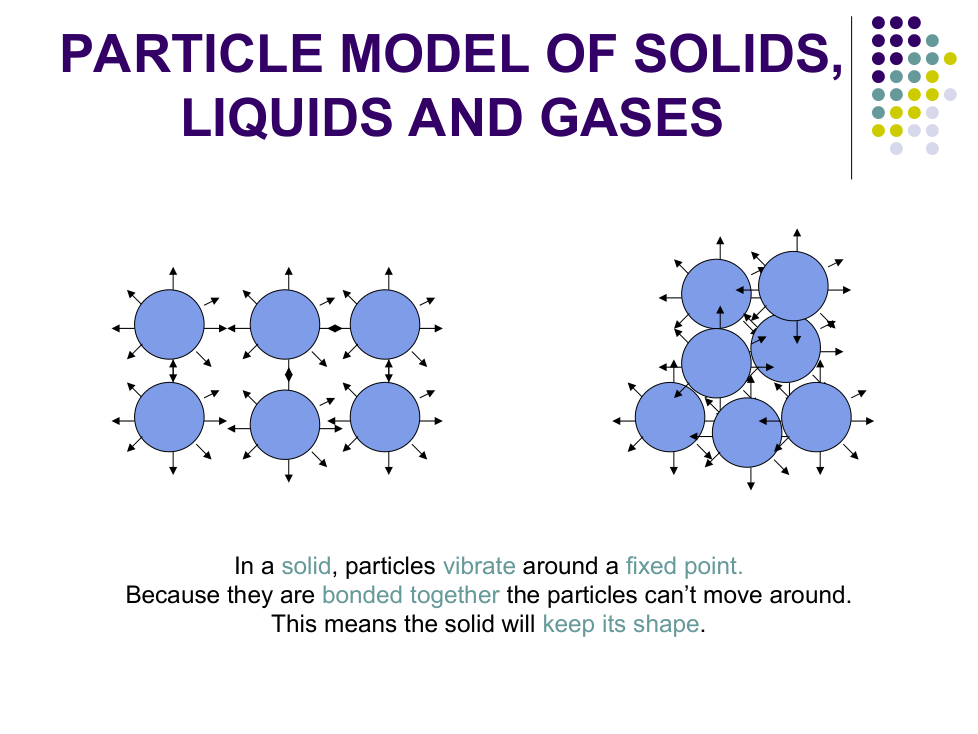
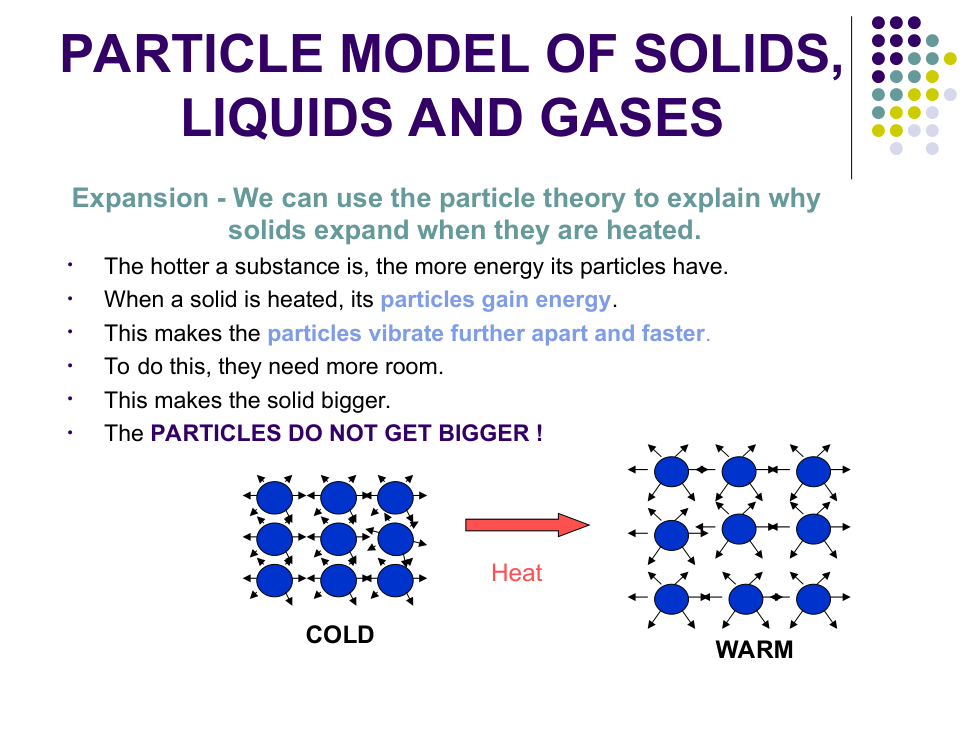
b) Why do solids not take the shape of their container?
Particles cannot _________ around
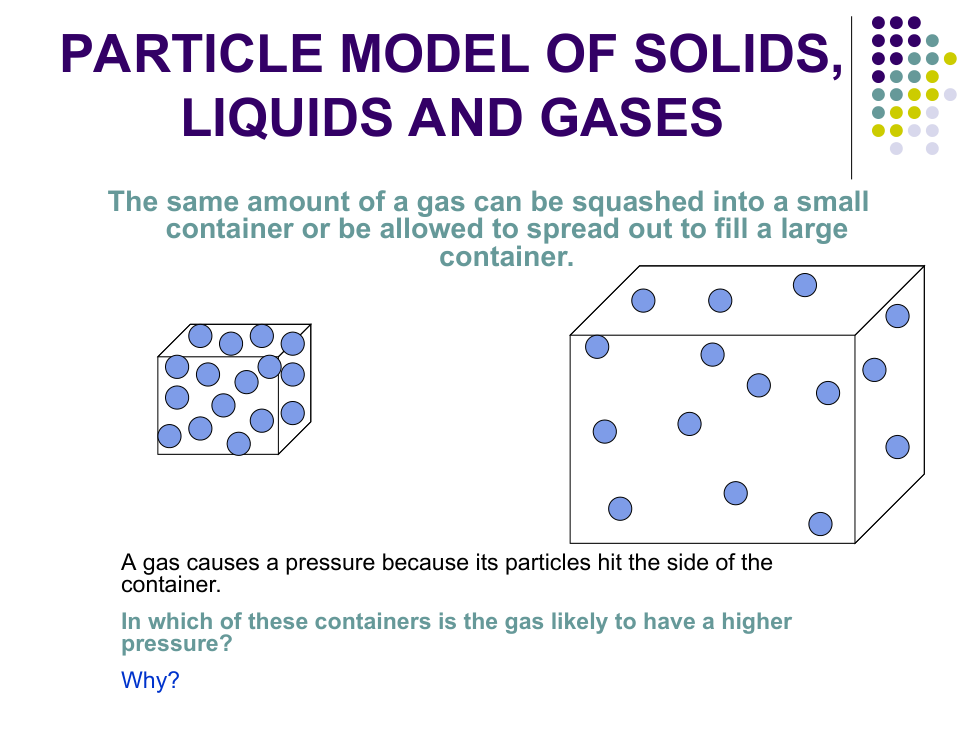
c) Why can gases be squashed easily?
There are big ___________ between the particles which can be pushed ___________ together
d) Why can't solids and liquids be squashed easily?
Particles are __________ so cannot be pushed ___________ together