Week 19 Year 11 Rates of Reaction 2
star
star
star
star
star
Last updated about 1 year ago
5 questions
1
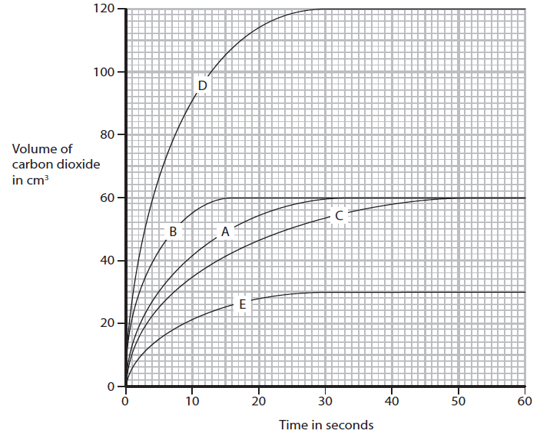
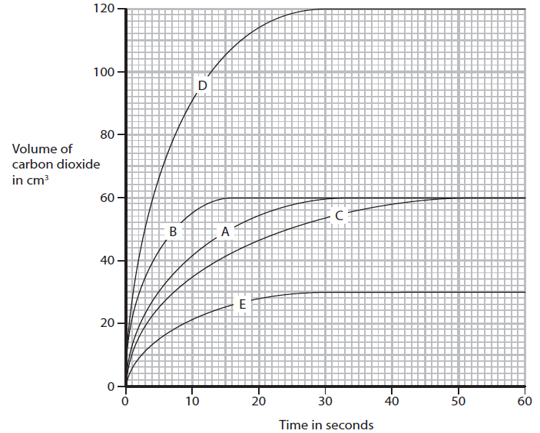
The rate of a reaction between hydrochloric acid and calcium carbonate can be increased by heating the solution. Explain why, using ideas about collisions between particles.
The rate of a reaction between hydrochloric acid and calcium carbonate can be increased by heating the solution. Explain why, using ideas about collisions between particles.
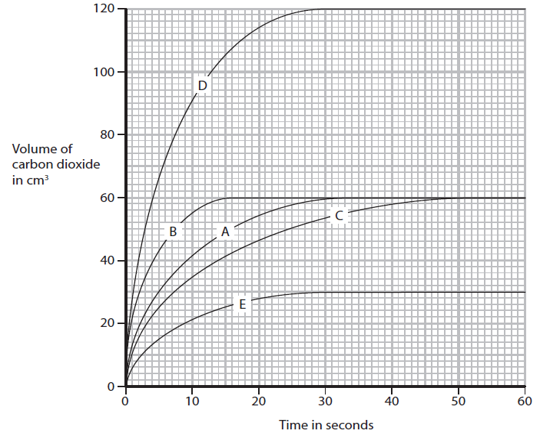
2