Year 10 Fuels lesson 1 (notes and practice)
star
star
star
star
star
Last updated 7 months ago
29 questions
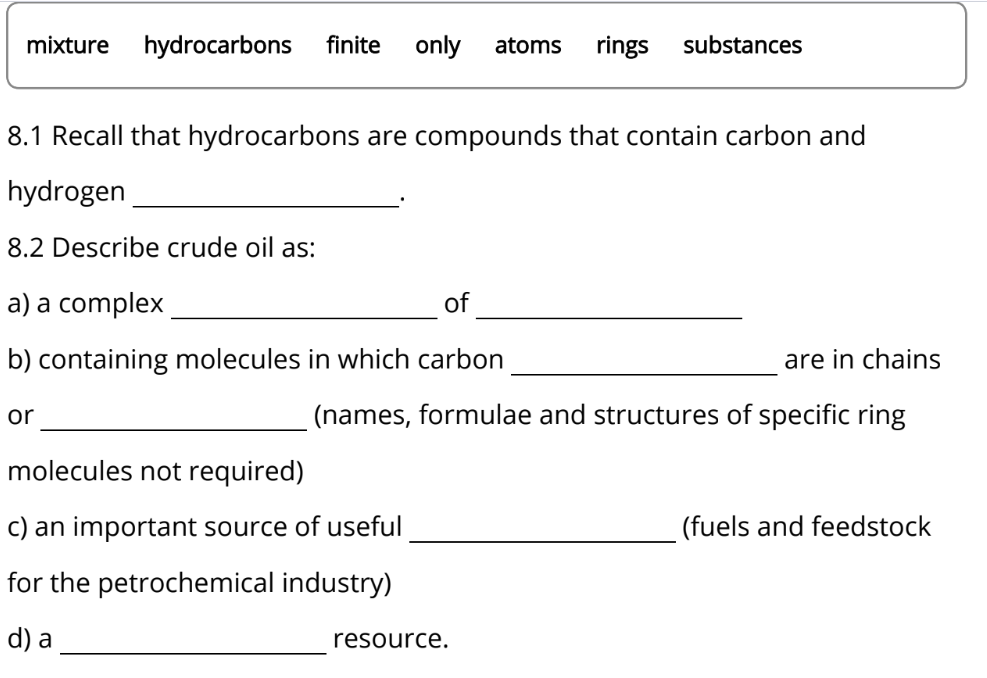
2
2
5
5
1
1
6
6
4
1
6
1
1
8
7
3
3
1
4
1
1
1
1
1
1
1
1
2
4
| Draggable item | arrow_right_alt | Corresponding Item |
|---|---|---|
petrol | arrow_right_alt | used in domestic heating and cooking |
diesel oil | arrow_right_alt | used as fuel for cars |
gases, | arrow_right_alt | used as fuel for aircraft |
fuel oil, | arrow_right_alt | used as fuel for some cars and trains |
kerosene | arrow_right_alt | used as fuel for large ships and in some power stations |
bitumen | arrow_right_alt | used to surface roads and roofs . |
Soot (carbon) | Water (0% in dry air) | Oxygen (21%) | Carbon dioxide (0.03%) | |
|---|---|---|---|---|
Which gas in the air reacts with the hydrocarbon? | ||||
The gas that condenses and collects in the U tube is | ||||
The gas that makes the limewater go cloudy is | ||||
Deposits of ________________ would form on the funnel |
Methane | poly(ethene) | Ethane | ethene | |
|---|---|---|---|---|
Option 1 | ||||
Option 2 | ||||
Option3 | ||||
Option 4 |