Law of Conservation of Mass Practice
star
star
star
star
star
Last updated 12 months ago
15 questions
Required
0
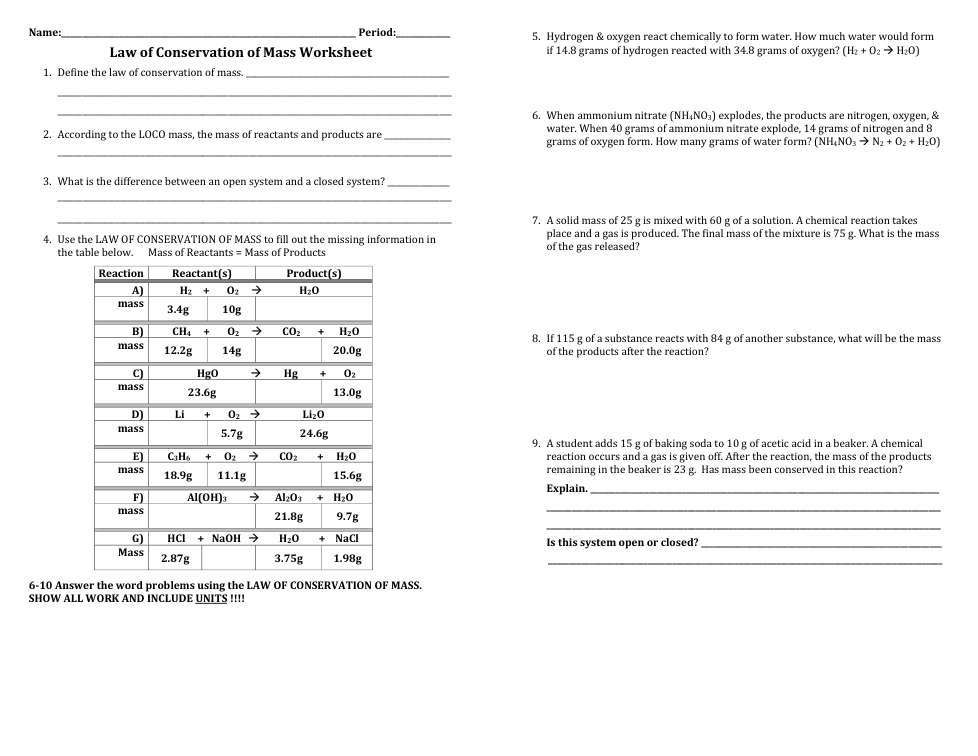
| Draggable item | arrow_right_alt | Corresponding Item |
|---|---|---|
open system | arrow_right_alt | systems that can result in a lower mass of the reactants in the products |
closed system | arrow_right_alt | systems that always result in an equal mass of the reactants in the products |