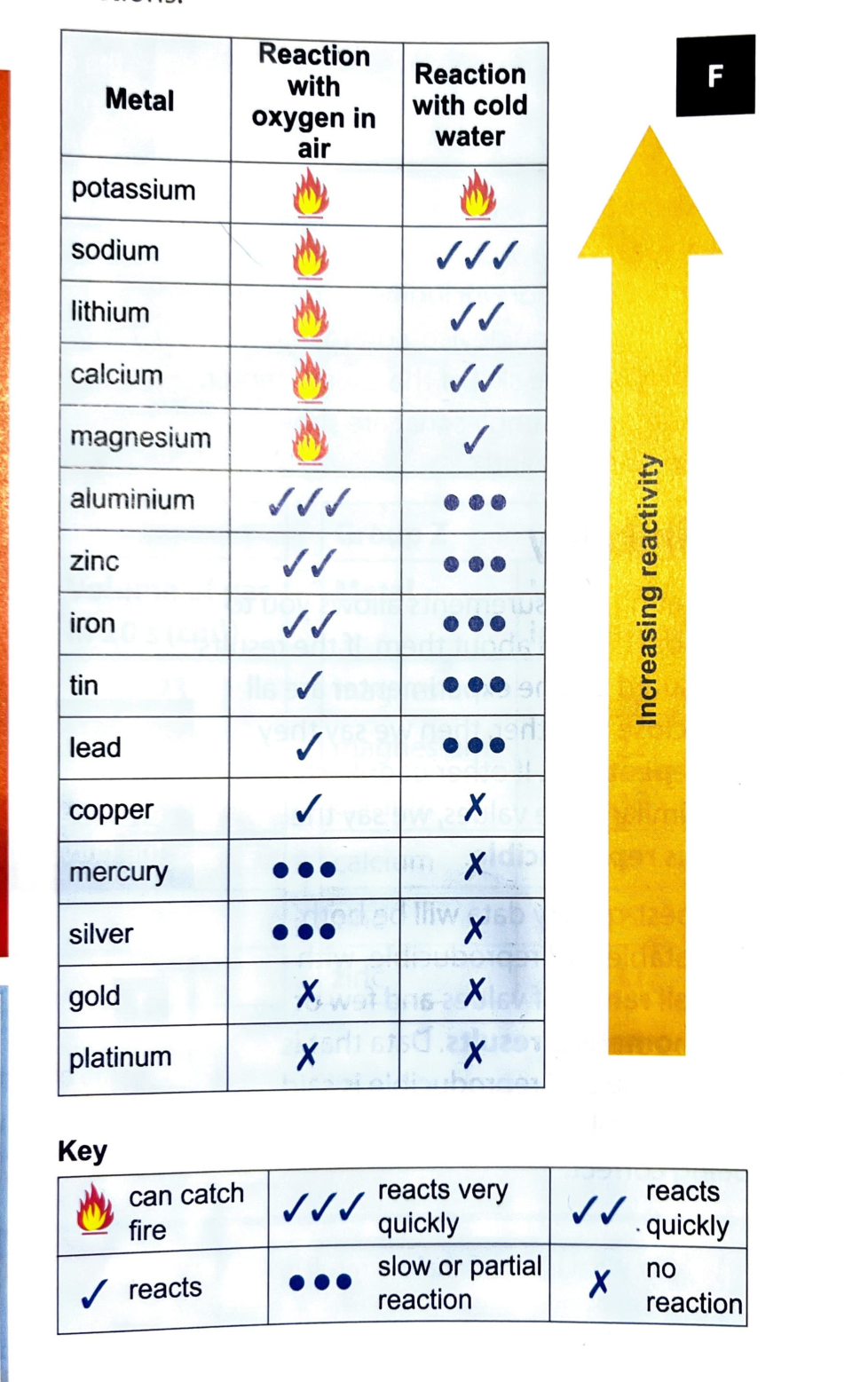
8Gc Metals & water
star
star
star
star
star
Last updated 5 months ago
12 questions
5

2
This reaction shows sodium and water. Name the products of this reaction
This reaction shows sodium and water. Name the products of this reaction
1

| Draggable item | arrow_right_alt | Corresponding Item |
|---|---|---|
Lithium | arrow_right_alt | releases hydrogen gas, moves around the container |
Rubidium | arrow_right_alt | releases hydrogen gas, moves around the container quickly, forms a ball |
Sodium | arrow_right_alt | releases hydrogen gas, lilac flame |
Potassium | arrow_right_alt | violent reaction, hydrogen gas released very quickly |
Caesium | arrow_right_alt | very violent reaction, hydrogen gas is released very quickly |