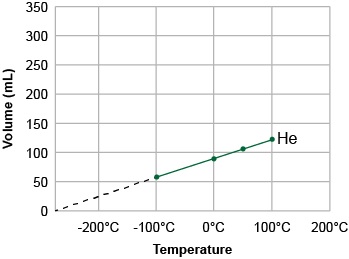
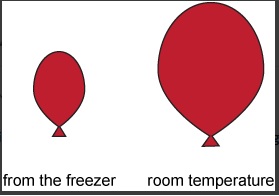
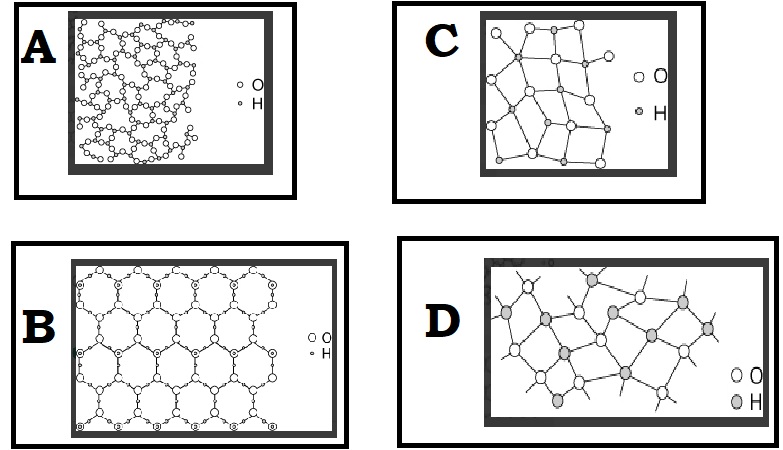
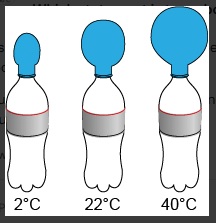
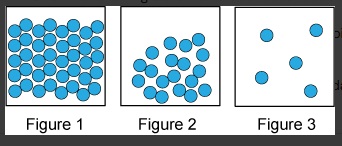
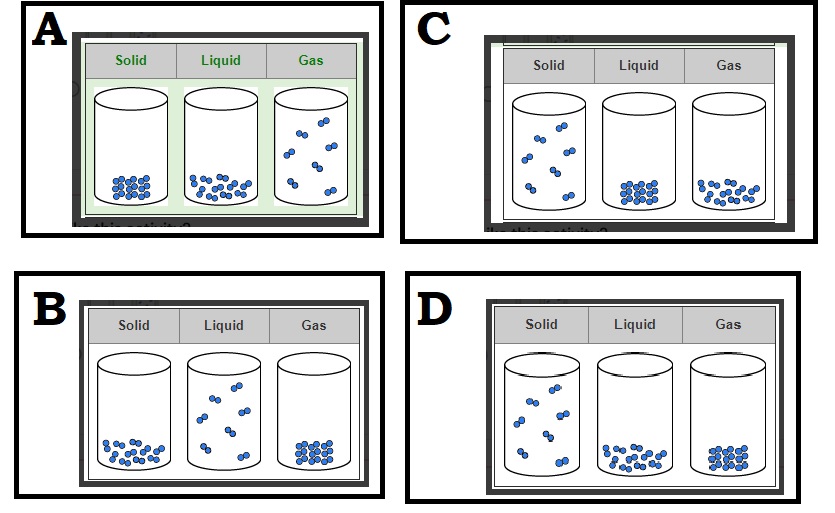
Unit 1 Test: Properties of Matter
star
star
star
star
star
Last updated 6 months ago
41 questions
Required
2.5
Required
2.5
Required
2.5
Required
2.5
Required
2.5
Required
2.5
Required
2.5
Required
2.5
Required
2.5
Required
2.5
Required
2.5
Required
2.5
Required
2.5
Required
2.5
Required
2.5
Required
2.5
Required
2.5
Required
2.5
Required
2.5
Required
2.5
Required
2.5
Required
2.5
Required
2.5
Required
2.5
Required
2.5
Required
2.5
Required
2.5
Required
2.5
Required
2.5
5