1. Put 5cm3 of dilute HCl in three different _______________
2. add the metal samples to the HCl
3. Note the rate of _____________
volume of hydrochloric acid
same mass of metal (we will look into this further in Year 10)
same particle size of metal
no bubbles for ___________
magnesium bubbles ____________________ than iron
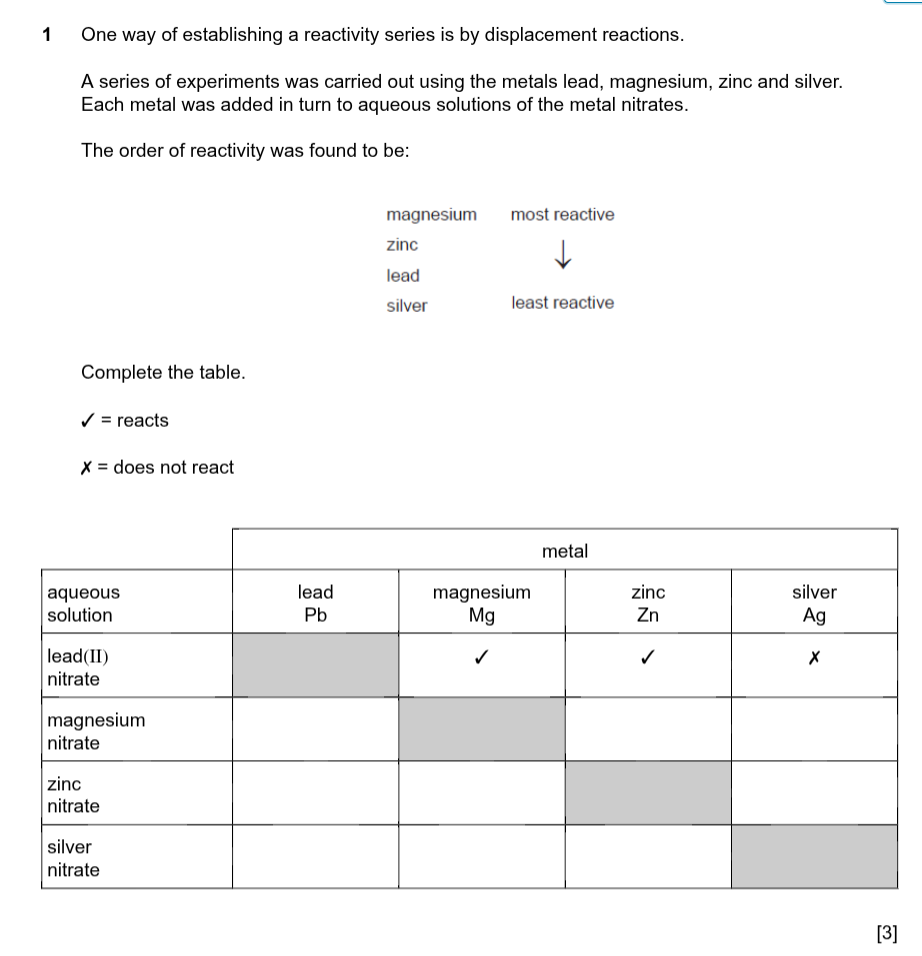
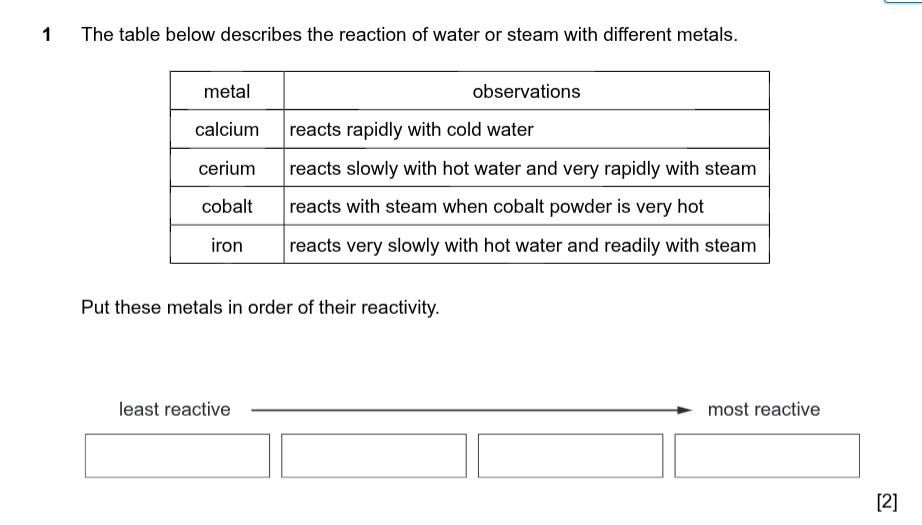
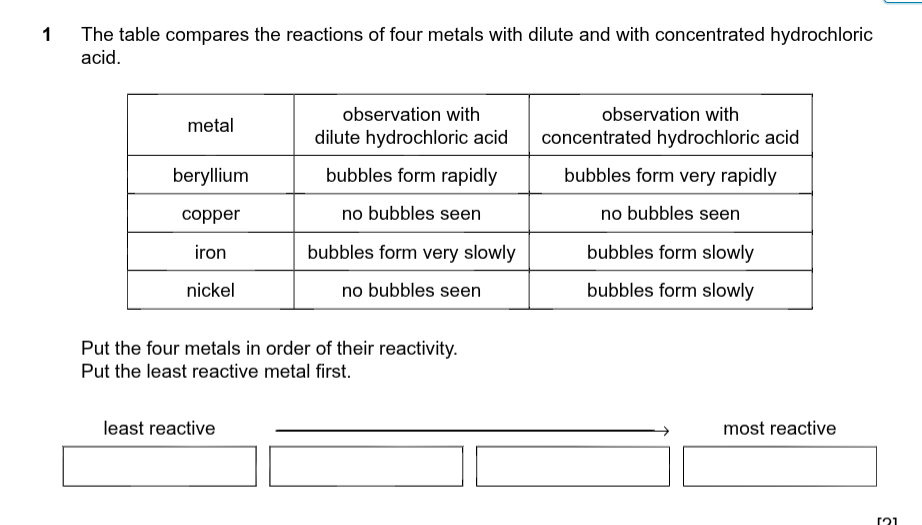
Reactivity series from least to most reactive is
Least reactive _______ ______________ Most reactive