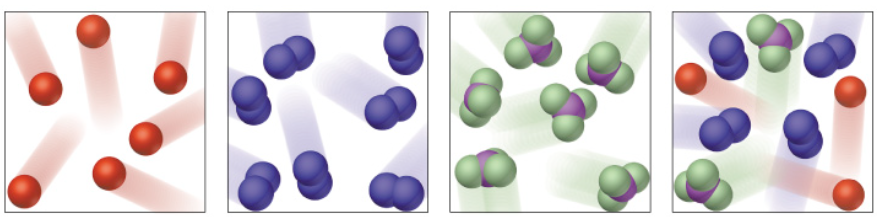
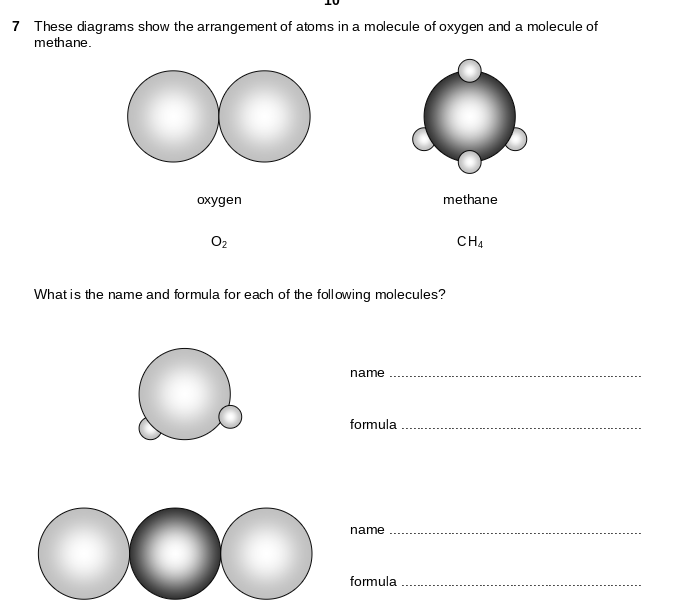
This exercise includes extra detail that you do not need to know until GCSE, (focus on the using the classification system).
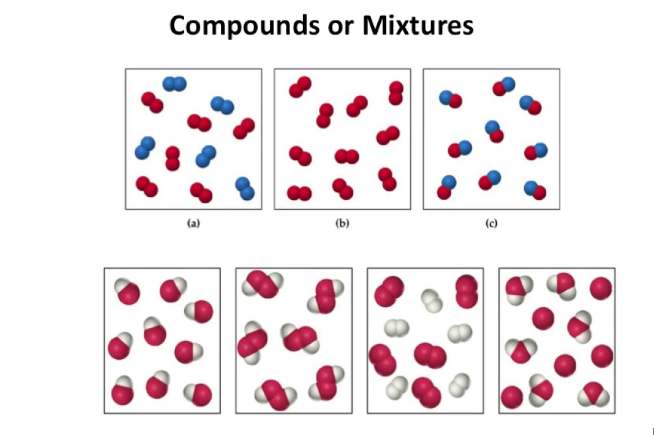
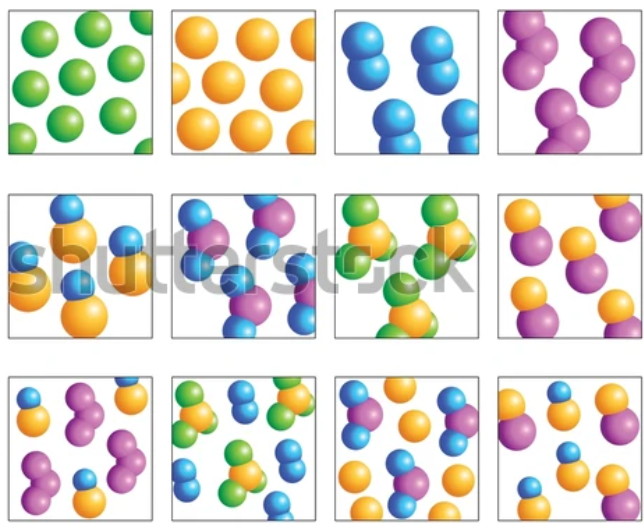
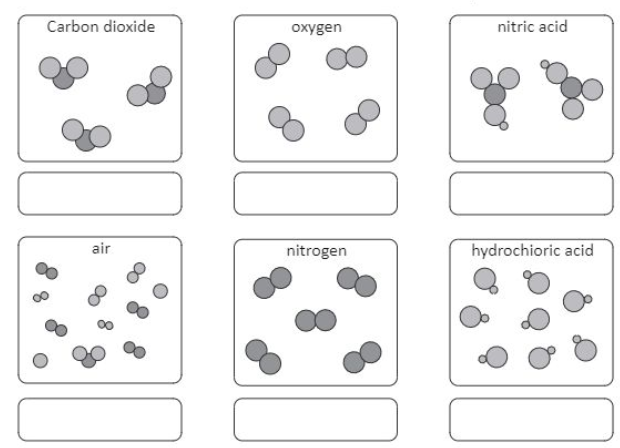
Elements have one type of atom only
Elements can be found on the periodic table
Elements have one capital letter in their formula only
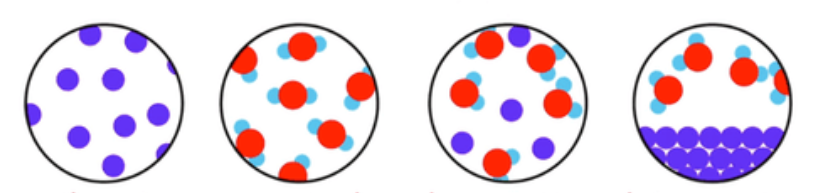
Compounds have more than one type of element chemically joined together in a fixed ratio.
Compounds have a formula with more than one capital letter.
Compounds can not be found on the periodic table.
Mixtures have more than one type of particle, jumbled together.
The particles do not have to be in a fixed ratio.
e.g.1. inhaled and exhaled air have different percentages of gases.
e g.2. Tea can be weak or strong, contain milk or not and contain sugar or not.