C1 Atomic Structure and Periodic Table
star
star
star
star
star
Last updated about 1 month ago
236 questions
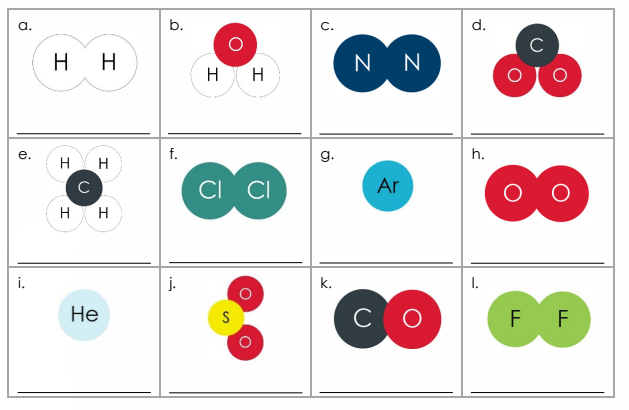
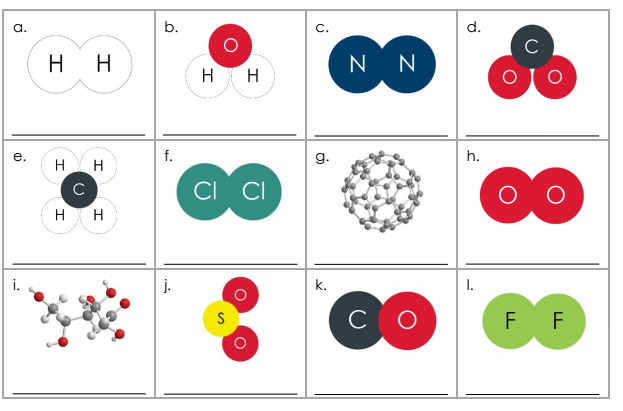
4.1.1.1 Atoms, Elements, and compounds
1
1
Required
1
Required
1
Required
1
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
1
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
1
1
1
1
4.1.1.2 Mixtures
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
Required
1
Required
1
1
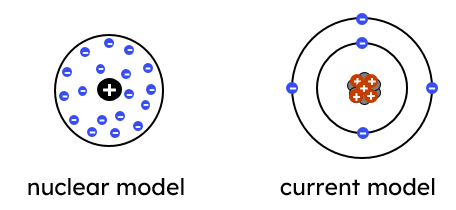
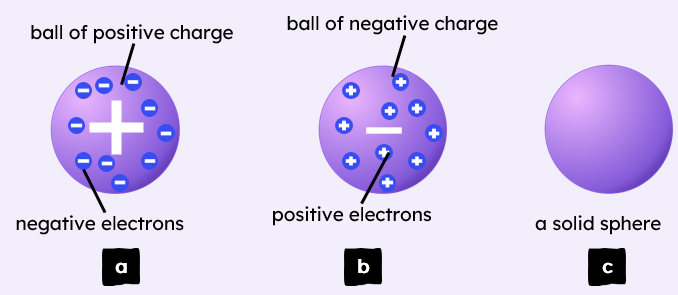
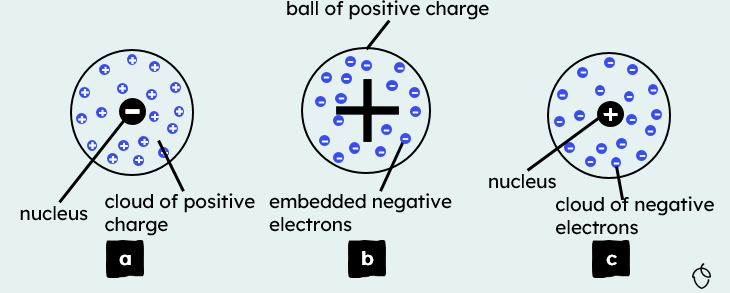
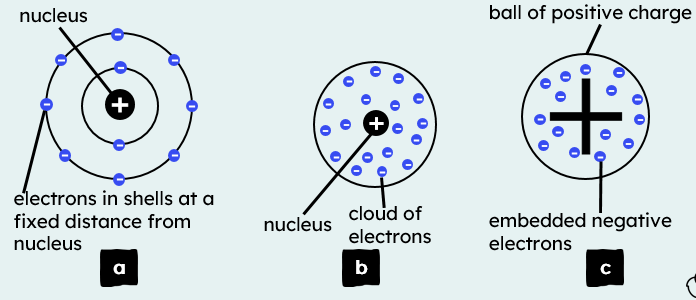
4.1.1.3 The Development of the model of the atom
1
4
1
Required
1
5
1
1
1
1
1
1
1
1
1
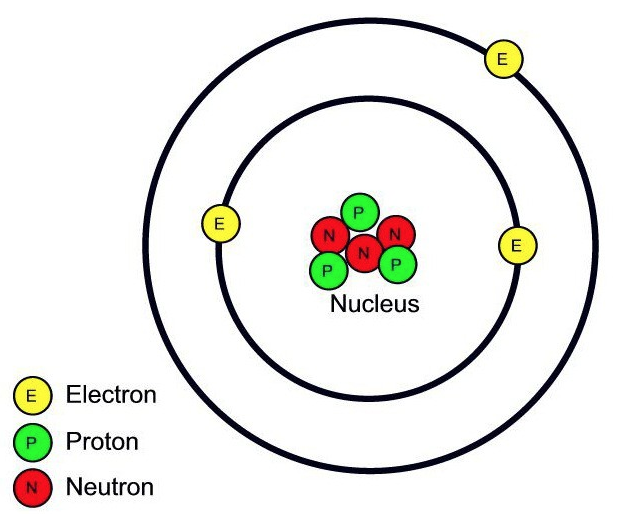
4.1.1.4 Relative electrical charges of subatomic particles
1
1
1
1
1
1
1
1
1
1
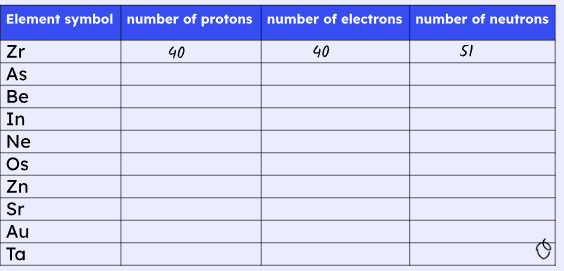
4.1.1.5 Size and mass of atoms
1
1
1
2
Required
1
Required
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
3
1
1
Required
1
Required
1
Required
1
Required
1
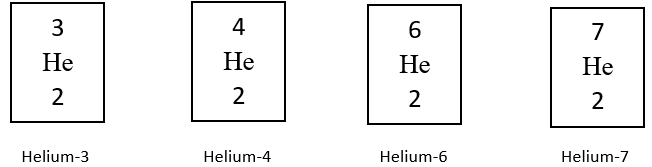
4.1.1.6 Relative Atomic Mass
1
1
1
1
1
1
1
1
1
1
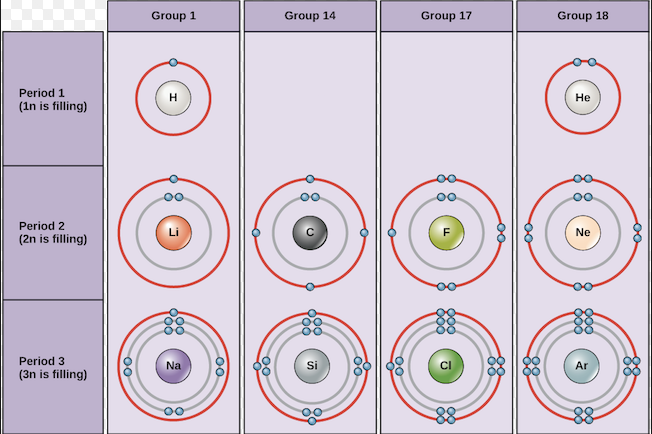
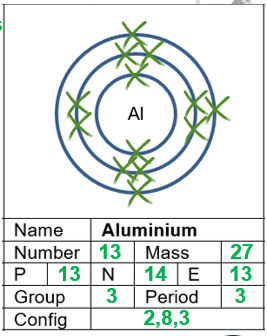
4.1.1.7 Electronic Structure
1
1
1
1
1
Required
1
Required
1
1
Required
1
Required
1
Required
1
Required
1
1
1
1
1
1
Required
1
Required
1
Required
1
Required
1
Required
1
1
1
1
4.1.2.1 The Periodic Table
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
4.1.2.2 Development of the Periodic Table
1
1
1
1
4.1.2.3 Metals and non-metals
1
1
4.1.2.4 Group 0
Required
1
Required
1
Required
1
1
1
1
1
1
4.1.2.5 Group 1
1
1
1
1
1
Required
1
Required
1
Required
1
Required
1
Required
2
4.1.2.6 Group 7
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
1
1
4
1
1
4.1.3 Properties of Transition Metals
1
1
1
1
Required
1
1
1