C2 Bonding, structure, and the properties of matter
star
star
star
star
star
Last updated 25 days ago
166 questions
4.2.1.1 Chemical Bonds
4
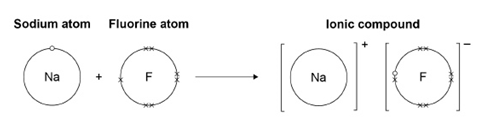
4.2.1.2 Ionic Bonding
Required
1
Required
1
Required
1
Required
1
Required
1
1
Required
1
Required
1
1
1
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
5
5
1
Required
1
Required
1
1
Required
1
Required
1
Required
1
1
1
1
1
4.2.1.3 Ionic Compounds
1
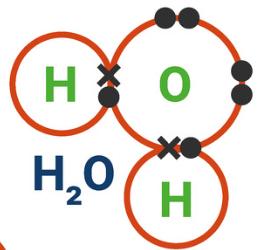
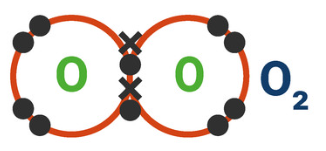
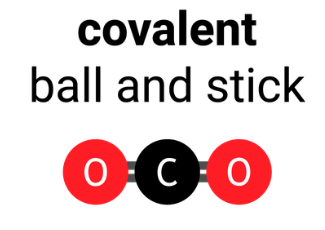
4.2.1.4 Covalent Bonding
1
1
2
1
1
1
3
1
2
2
1
1
1
1
1
4.2.1.5 Metallic Bonding
1
1
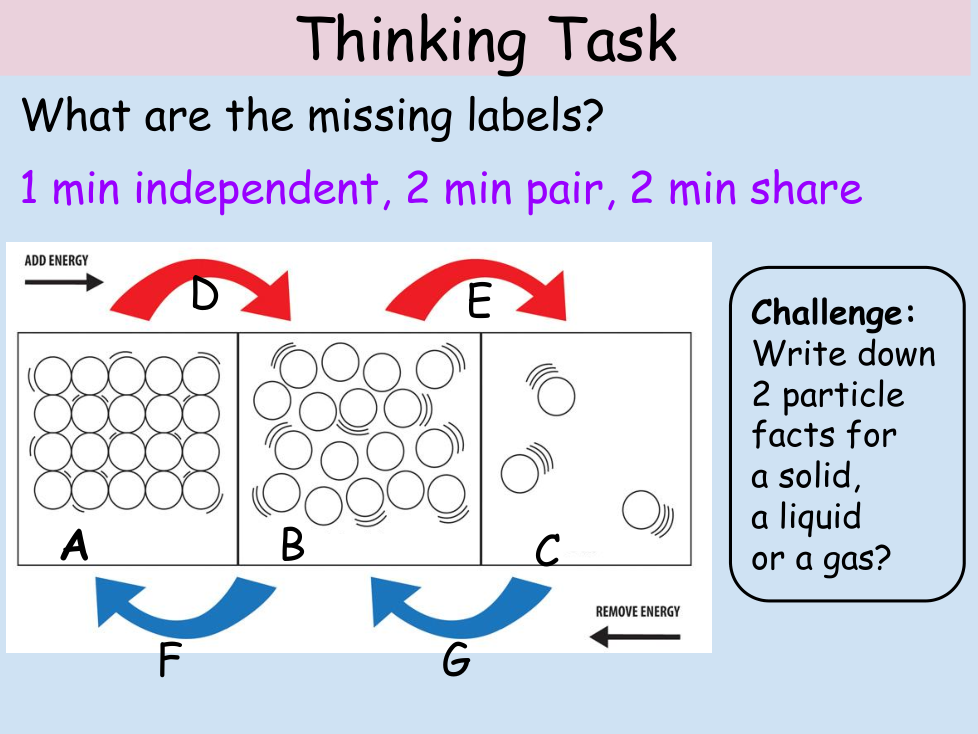
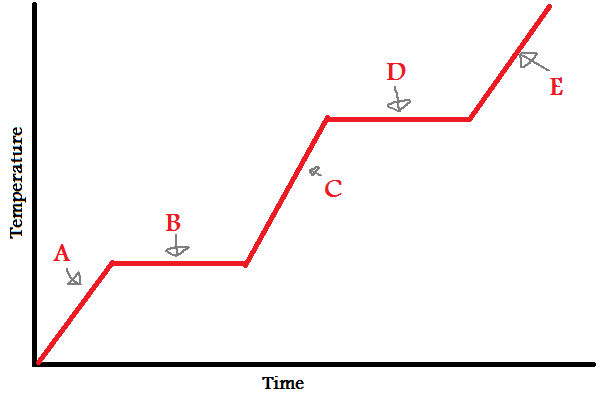
4.2.2.1 The Three states of Matter
2
2
2
1
1
1
1
1
9
20
20
10
10
10
1
1
4.2.2.2 State Symbols
1
1
1
4.2.2.3 Properties of Ionic Compounds
1
1
1
2
2
1
1
1
5
1
2
2
2
1
1
1
4.2.2.4 Properties of small molecules
1
1
1
1
4.2.2.5 Polymers
2
1
1
4.2.2.6 Giant Covalent Structures
6
6
3
1
1
1
4.2.2.7 Properties of Metals and Alloys and 4.2.2.8 Metals as Condudctors
1
1
1
1
1
1
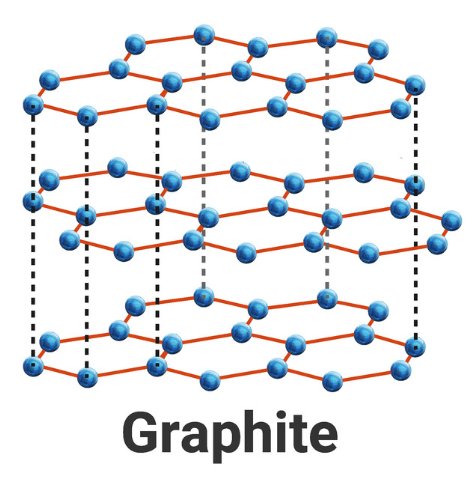
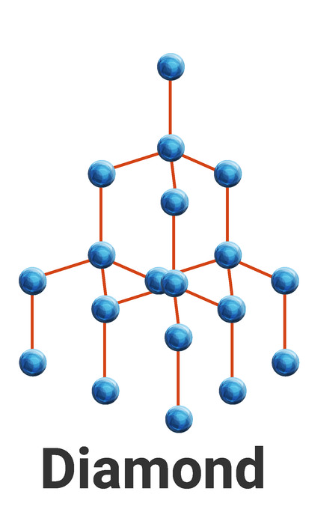
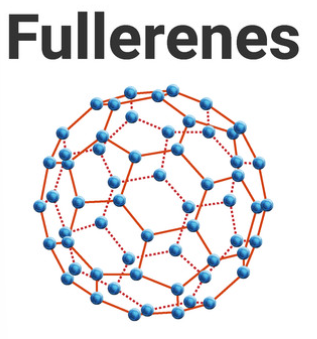
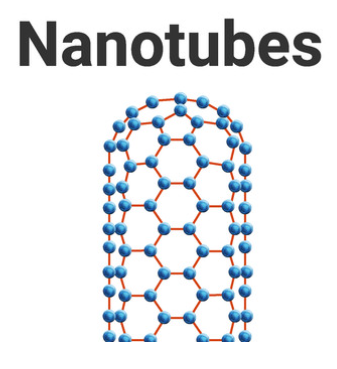
4.2.3 Structure and bonding of carbon
1
1
1
1
…
1
1
1
1
1
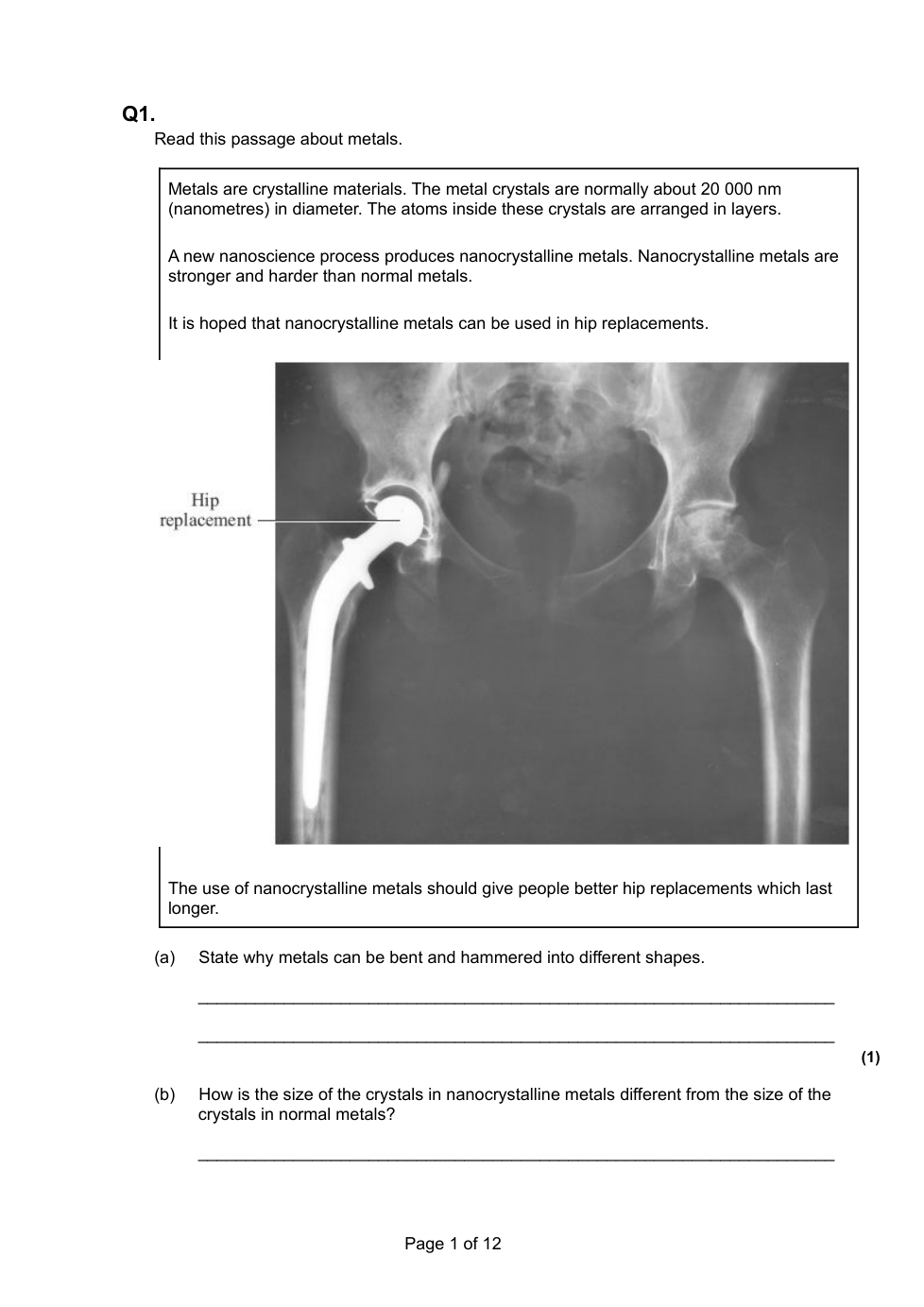
4.2.4 Bulk and surface properties of matter including nanoparticles
1
1
1
1
6
1
1
6
6
3