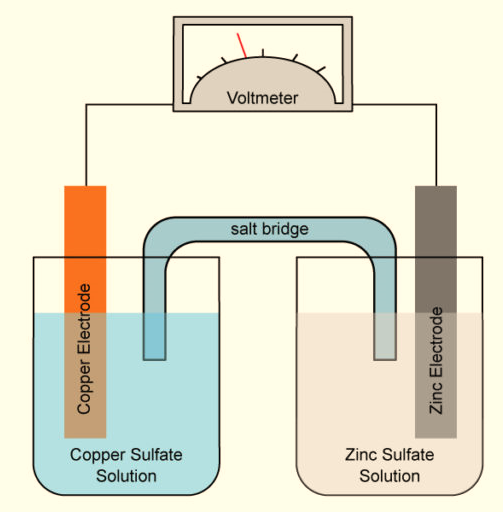
A student investigated the temperature change when zinc reacts with copper sulfate solution.
The student used a different concentration of copper sulfate solution for each experiment.The student used the apparatus shown below.
• measured 50 cm3 copper sulfate solution into a glass beaker
• measured the temperature of the copper sulfate solution
• measured the highest temperature
• repeated the experiment using copper sulfate solution with different concentrations.
The equation for the reaction is:
Zn(s) + CuSO4(aq) --> Cu(s) + ZnSO4(aq)
zinc + copper sulfate solution --> copper + zinc sulfate solution
The student’s results are shown in the table below.
Describe and explain the trends shown in the student’s results.