C7 Organic Chemistry
star
star
star
star
star
Last updated 25 days ago
59 questions
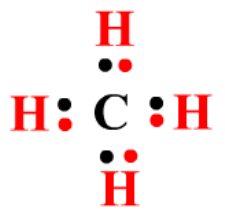
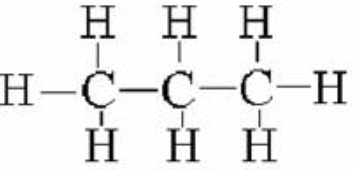
4.7.1.1 Crude oil, hydrocarbons and alkanes
1
1
7
1
1
1
1
1
1
5
1
1
1
1
1
2
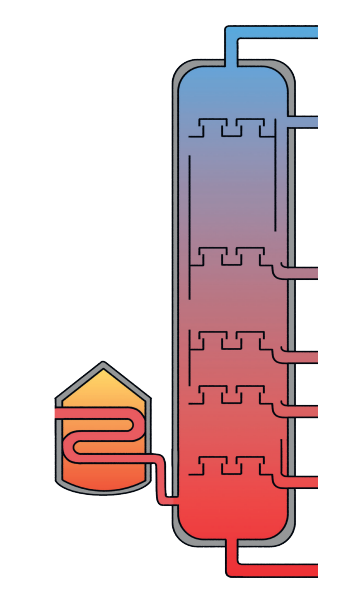
4.7.1.2 Fractional distillation and petrochemicals
1
2
1
1
1
3
4
1
1
1
1
1
1
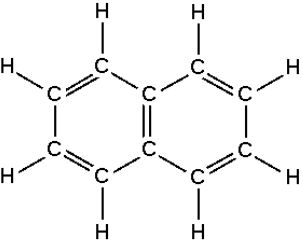
4.7.1.3 Properties of hydrocarbons
8
4
9
6
1
4.7.1.4 Cracking and alkenes
1
1
1
1
1
1
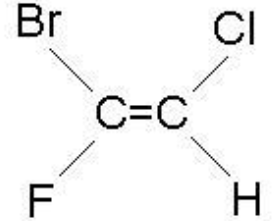
4.7.2.1 Structure and formulae of alkenes
4.7.2.2 Reactions of alkenes
1
1
1
1
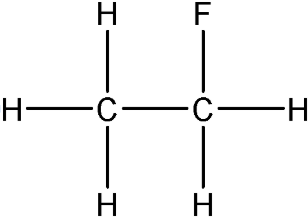
4.7.2.3 Alcohols
1
1
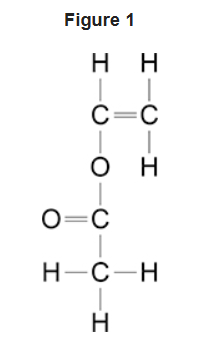
4.7.2.4 Carboxylic acids
1
1
4.7.3 Synthetic and naturally occurring polymers
1
1
1
1
1
1