C3 Quantitative Chemistry
star
star
star
star
star
Last updated 25 days ago
193 questions
4.3.1.1 Conservation of mass and balanced chemical equations
2
2
2
2
2
1
1
1
1
1
1
3
3
3
3
4
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
5
1
3
2
1
4
1
1
1
1
1
2
2
2
2
2
1
1
1
4.3.1.2 Relative Formula Mass
2
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
4.3.1.3 Mass changes when a reactant or product is a gas
4.3.1.4 Chemical measurements
4.3.2.1 Moles
2
1
1
1
1
1
1
1
1
1
1
1
1
1
4.3.2.2 Amounts of substances in equations
1
1
1
1
1
1
1
1
1
4.3.2.3 Using moles to balance equations
1
1
1
1
1
1
4.3.2.4 Limiting reactants
1
1
1
1
1
1
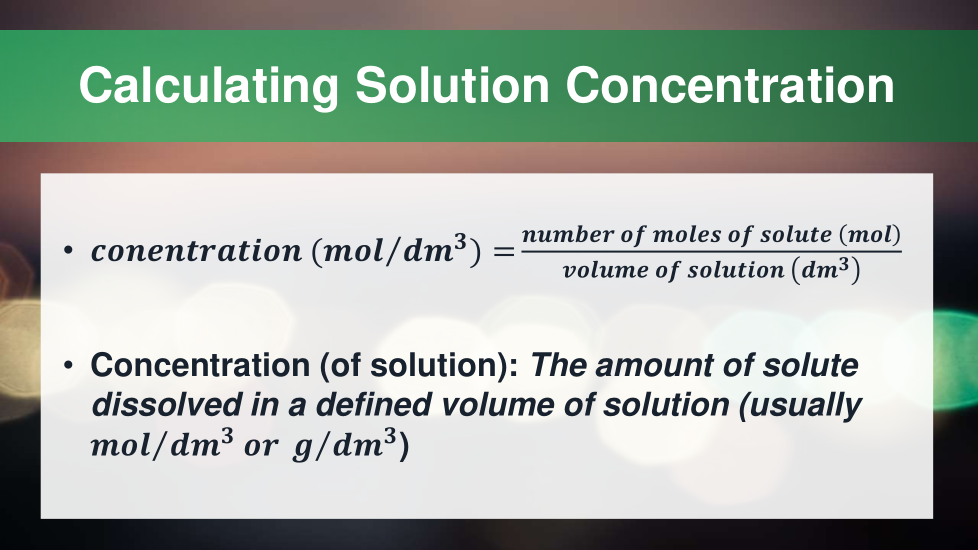
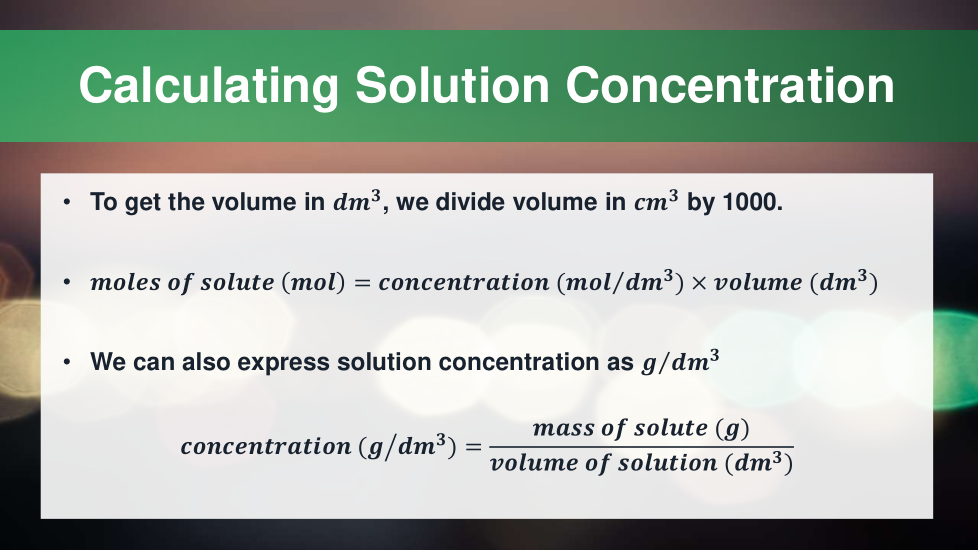
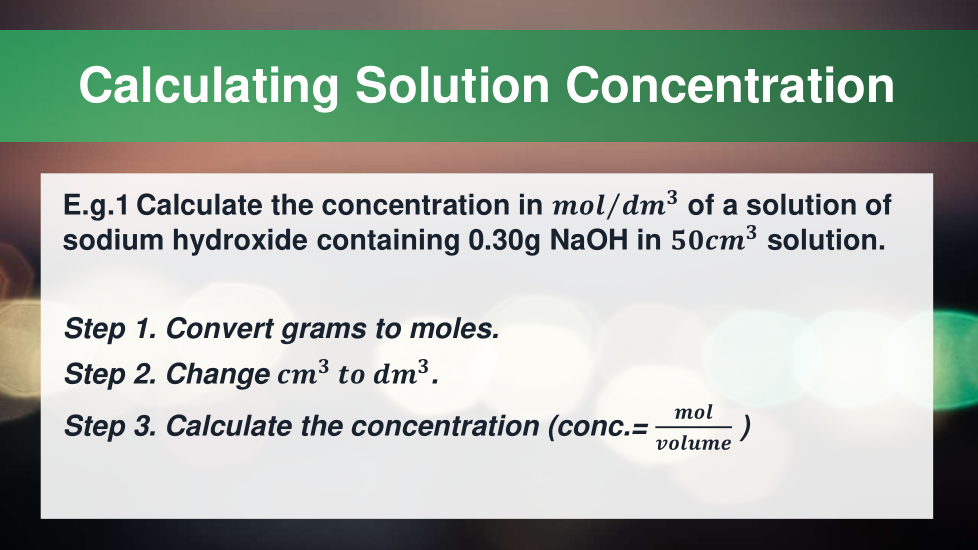
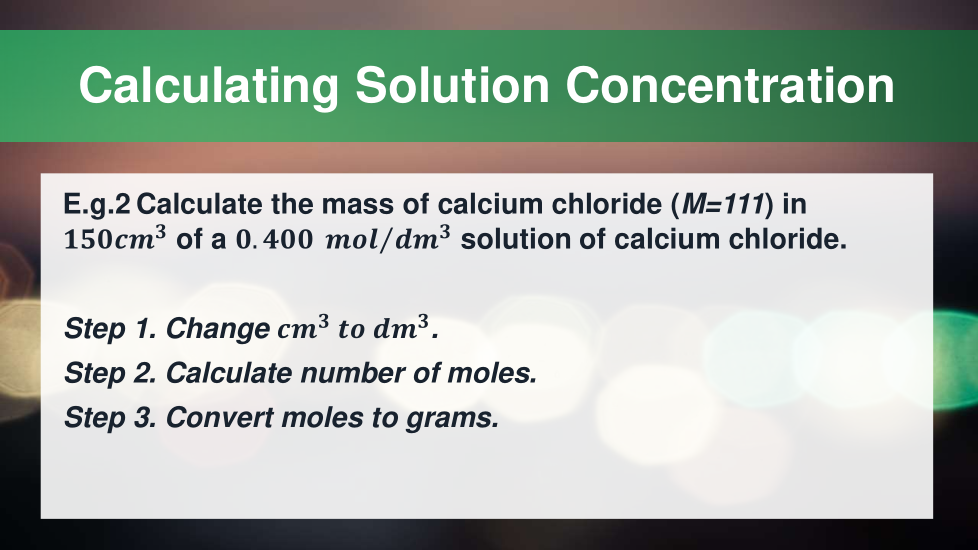
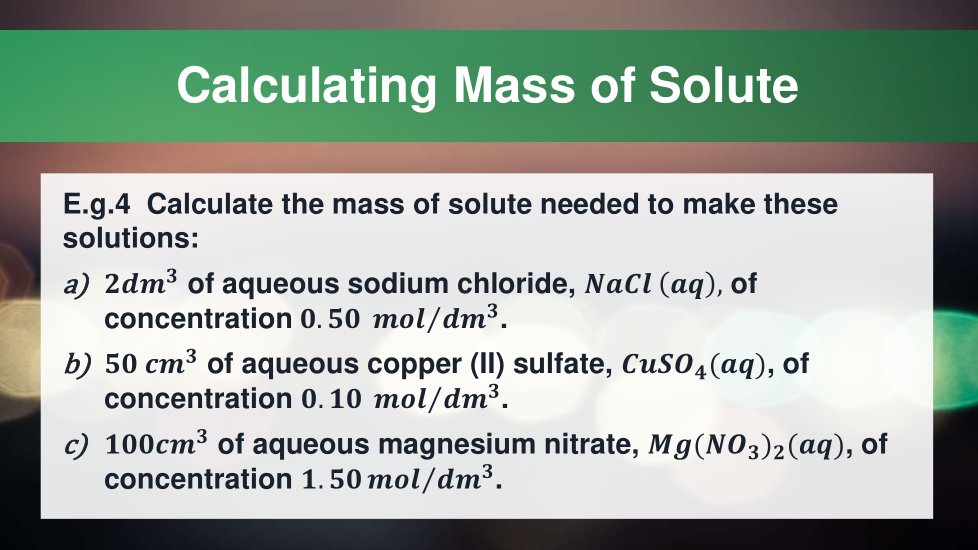
4.3.2.5 Concentration of solutions
1
1
1
1
1
1
1
1
9
4.3.3.1 Percentage yield
1
1
1
1
1
1
1
1
1
1
1
1
1
1
4.3.3.2 Atom economy
1
1
1
1
1
1
1
1
1
1
1
1
4.3.4 Using concentrations of solutions in mol/dm 3
4.3.5 Use of amount of substance in relation to volumes of gases
1
1
1
1
1
1
1
1
1
1
1
1