C8 Gas Tests and Separating Mixtures FT (05/02/2026)
star
star
star
star
star
Last updated 14 days ago
20 questions
4.8.1.1 Pure substances
Required
1
Required
1
Required
1
Required
1
2
Required
1
Required
1
1
Required
1
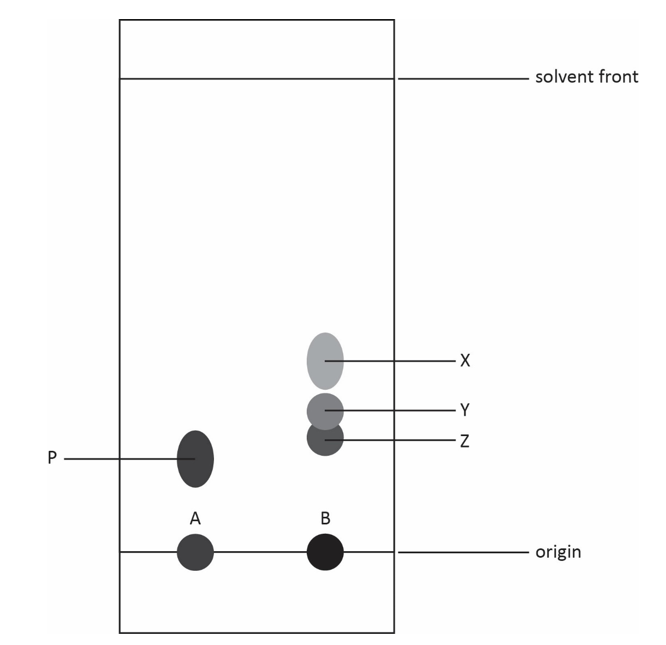
4.8.1.3 Chromatography
1
1
1
1
1
2
8
4.8.2 Identification of common gases
1
…