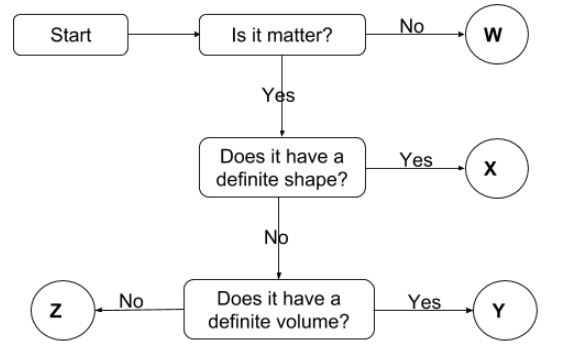
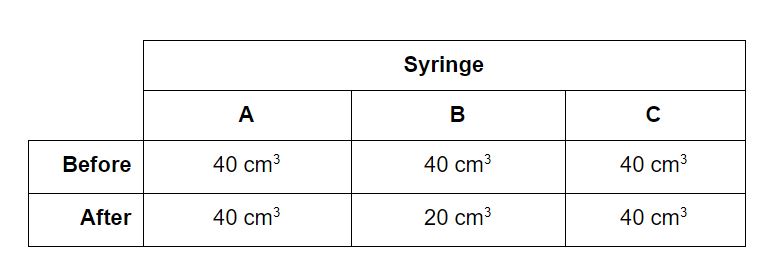
Particulate nature of matter
star
star
star
star
star
Last updated over 7 years ago
15 questions
Note from the author:
Introduction to the particulate nature of matter. After introducing the concepts through videos and a ChemThink interactive, students must apply what they've learned to answer a couple of questions.
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1