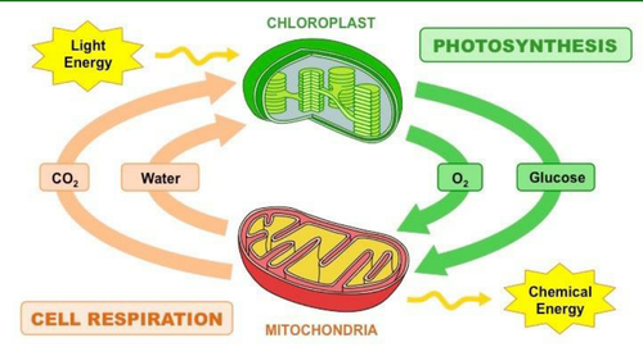
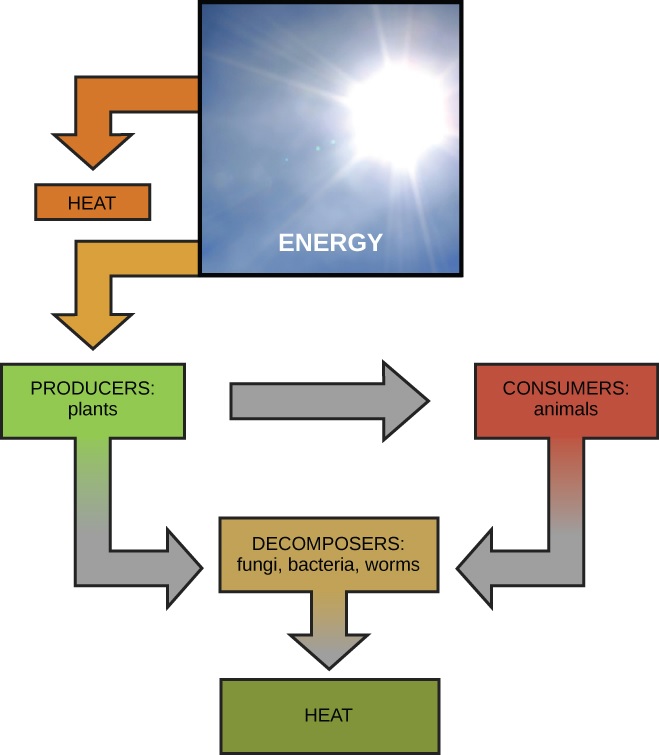
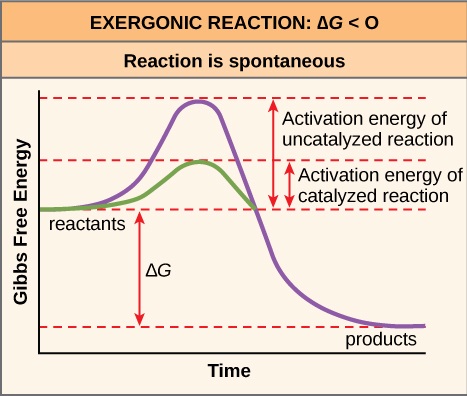
Cell Metabolism Part 1 Concept Review
star
star
star
star
star
Last updated almost 8 years ago
16 questions
Note from the author:
A review assignment examining the foundational concepts of energy and energy stored in chemical bonds.
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1