Once the physical and chemical properties of matter are determined, those properties aid scientists in classifying matter into about six different categories. To determine which of the six different categories the particular matter that is under investigation belongs to, it is helpful to ask a series of questions:
1. Is the matter uniform in composition?
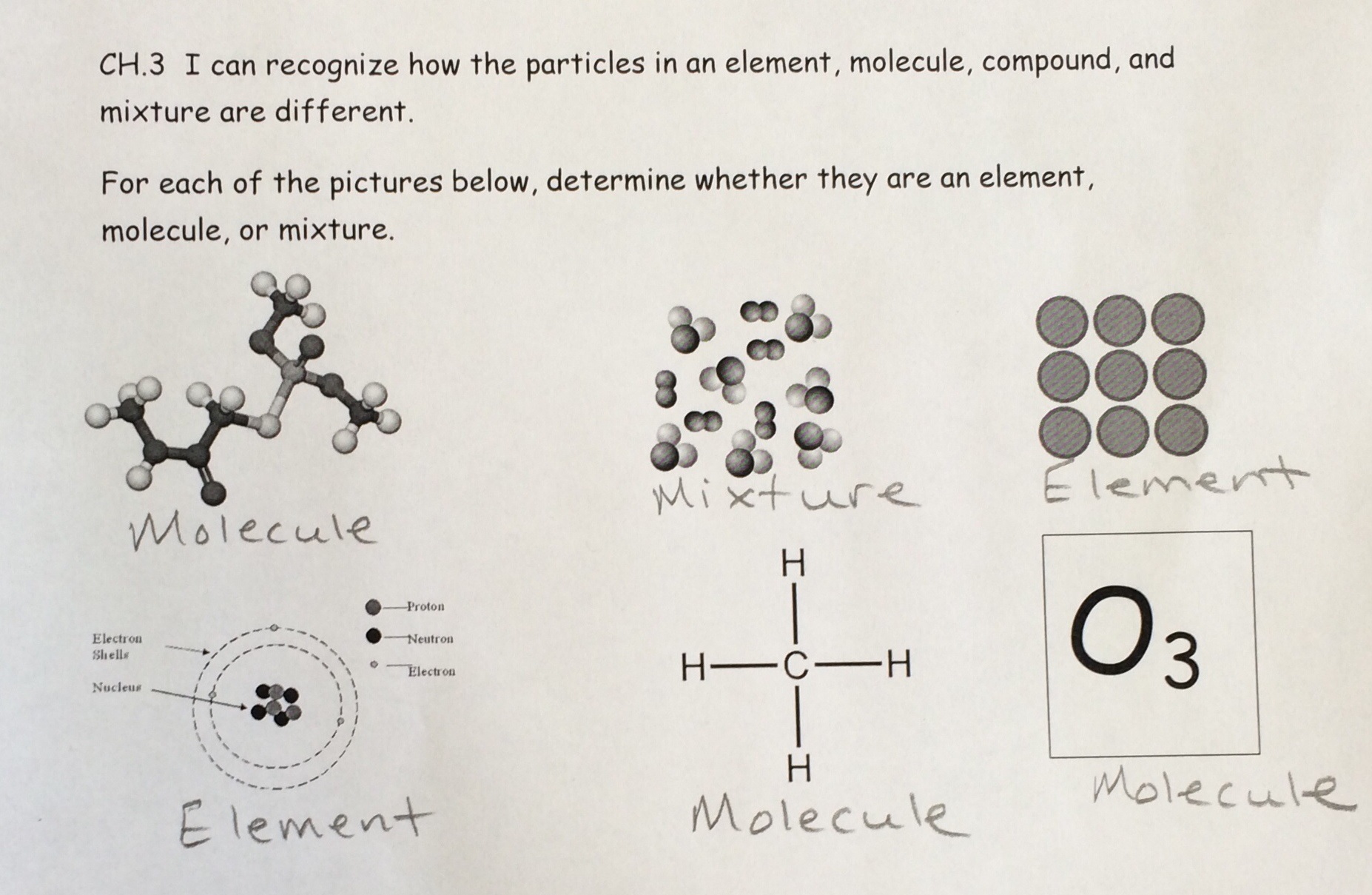
To be uniform in the composition, matter must be made up of all the same atoms, or different atoms always combined in the same ratio. If matter is uniform in composition, then it is classified as a pure substance. If matter is not uniform in composition (different atoms in different ratios), then it is classified as a mixture of pure substances.
Because a mixture is a physical mix of pure substances, it must be separable by physical means, as in distillation, chromatography, using a magnet, filtration, or even using your fingers.
2. If the matter is a substance, is it composed of one single type of atom?
Pure substances are uniform in composition, meaning it is either composed of only one single type of atom, called an element, or composed of different atoms always combined in the same ratio, called compounds. Compounds usually have very different properties than the elements that actually compose it. For example, sodium metal is explosive in water and chlorine gas is very poisonous, but the compound of sodium chloride is a white, crystalline solid that is necessary for the human body.
Elements cannot be broken down any further by chemical means. It takes nuclear reactions to break open an atom. However, compounds can be broken down into the elements that compose it by chemical means.
3. If the matter is a mixture, is the mixing uniform?
Mixtures of pure substances are classified by the extent that the mixture is uniformly composed. There are two types of mixtures; homogeneous and heterogeneous. The Greek words “homo” means “same” and “hetero” means “different”. Thus, a homogeneous mixture is where all components are in the same phase, like in a well-made glass of iced tea. In contrast, the components of a heterogeneous mixture are in different phases with an obvious boundary (interface) between the phases, like in fresh-squeezed orange juice where the pulp settles to the bottom.
Homogeneous mixtures are also called solutions, and if something is a solution, it must be a homogeneous mixture. Solutions have completely even mixing of the components, and do not necessarily have to be a liquid. Bronze is an example of a solid solution, and air is an example of a gaseous solution.
4. If the mixture is a solution, which parts are the solute and solvent?
When a homogeneous mixture is being made, least two pure substances are needed. One of the substances dissolves the other to form the solution. The substance being dissolved is called the solute, and the substance doing the dissolving is called the solvent. Sometimes it’s easy to tell which is which – to make lemonade, lemon juice and sugar are both dissolved by water so therefore the lemon juice and sugar are the solutes and water is the solvent. Other times it’s difficult to tell – ethyl alcohol will dissolve completely in any amount of water, and water will dissolve completely in any amount of ethyl alcohol. The rule of thumb is that the substance in greatest amount is considered the solvent. Anything else by default is considered solute.
5. If the mixture is heterogeneous, does it settle?
Heterogeneous mixtures have parts that are noticeably different because they are in different phases. Sometimes these parts settle, like a bucket of muddy water, and sometimes the different parts don’t settle, like smog.
If the particles are large enough that settling will occur, if given enough time, then the heterogeneous mixture is considered a suspension. Any food that states "Shake Well Before Using" is guaranteed to be a suspension.
If the particles do not settle after an appreciably amount of time, then it is a special type of suspension called a colloidal suspension.
When you observe a mixture and only one phase is present, then it is a solution; when two or more phases are present, then the mixture is considered to be heterogeneous. You can see the phases because they usually meet at a well-defined interface, oil and vinegar salad dressing.
One of the definitional ways to tell if a heterogeneous mixture is a colloid is using the Tyndall Effect. Colloids will scatter light, allowing a beam to be seen in the colloid; a solution will not show the beam of light; and often suspensions will completely block out the light.