Chapter 12 Test
star
star
star
star
star
Last updated over 5 years ago
50 questions
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
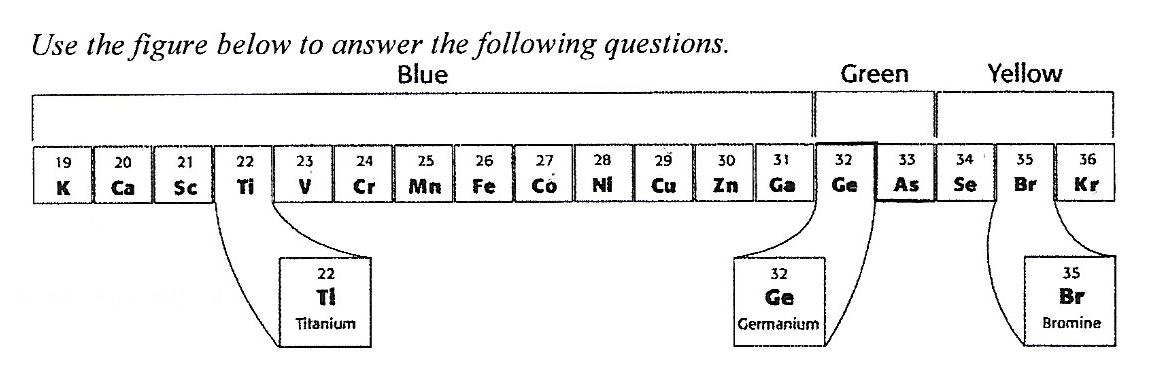
2
2
2

2
2
halogens, period, group, alkali metals, actinide, periodic law, periodic, noble gases, alkaline-earth metals
2
halogens, period, group, alkali metals, actinide, periodic law, periodic, noble gases, alkaline-earth metals
2
halogens, period, group, alkali metals, actinide, periodic law, periodic, noble gases, alkaline-earth metals
2
halogens, period, group, alkali metals, actinide, periodic law, periodic, noble gases, alkaline-earth metals
2
aluminum, oxygen, hydrogen, silicon, carbon
2
aluminum, oxygen, hydrogen, silicon, carbon
2
elements, periodic law, period, transition, periodic, group, alkali
2
elements, periodic law, period, transition, periodic, group, alkali
2
elements, periodic law, period, transition, periodic, group, alkali
2
elements, periodic law, period, transition, periodic, group, alkali
2
elements, periodic law, period, transition, periodic, group, alkali
2
alkali, lanthanides, metalloids, actinides, transition, alkaline-earth, halogens
2
alkali, lanthanides, metalloids, actinides, transition, alkaline-earth, halogens
2
alkali, lanthanides, metalloids, actinides, transition, alkaline-earth, halogens
2
alkali, lanthanides, metalloids, actinides, transition, alkaline-earth, halogens
2
2
2
2
2
2
2
2
2
2
2